��Ŀ����
6��A��B��C��D��E�����������ǿ�����ԭ�ӻ����ӣ�����������Ԫ�ض��ڶ����ڣ�A�Ը���ʱ�����ܱ��κ�����������������B��ԭ�Ӻ�����������ǰһ���ڵ�ͬ��Ԫ�ض�8���䵥�ʲ��ܴ�CuSO4��Һ���û���Cu��CԪ��������ͬλ��Cl��C2��C3��C1���������ֱ�ΪC2��C3��$\frac{1}{2}$��$\frac{1}{3}$��D����̬�⻯������ˮ���Լ��ԣ�E�������ֲ�ͬԪ����ɵĴ�����ɵ�����������10�����ӣ�E������C+��ϳ����������ش���1��д���������ķ��ţ�AF��BNa��CH��DN��EOH-��
��2��C��ͬλ�����ƣ�C2뮣�
��3��C��D�γɷ��ӵĵ���ʽΪ
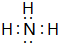 ���ռ�ṹΪ�����Σ�
���ռ�ṹΪ�����Σ�
���� A��B��C��D��E�����������ǿ�����ԭ�ӻ����ӣ�����������Ԫ�ض��ڶ����ڣ�A�Ը���ʱ�����ܱ��κ���������������AΪFԭ�ӣ�����B��ԭ�Ӻ�����������ǰһ���ڵ�ͬ��Ԫ�ض�8�����ڵ������ڣ��䵥�ʲ��ܴ�CuSO4��Һ���û���Cu����BΪNa��CԪ��������ͬλ��Cl��C2��C3��C1���������ֱ�ΪC2��C3��$\frac{1}{2}$��$\frac{1}{3}$����CΪHԭ�ӣ�D����̬�⻯������ˮ���Լ��ԣ���DΪNԭ�ӣ�E�������ֲ�ͬԪ����ɵĴ�����ɵ�����������10�����ӣ�E������H+��ϳ�����������EΪOH-���ݴ˽��
��� �⣺��1��A��B��C��D��E�����������ǿ�����ԭ�ӻ����ӣ�����������Ԫ�ض��ڶ����ڣ�A�Ը���ʱ�����ܱ��κ���������������AΪFԭ�ӣ�����B��ԭ�Ӻ�����������ǰһ���ڵ�ͬ��Ԫ�ض�8�����ڵ������ڣ��䵥�ʲ��ܴ�CuSO4��Һ���û���Cu����BΪNa��CԪ��������ͬλ��Cl��C2��C3��C1���������ֱ�ΪC2��C3��$\frac{1}{2}$��$\frac{1}{3}$����CΪHԭ�ӣ�D����̬�⻯������ˮ���Լ��ԣ���DΪNԭ�ӣ�E�������ֲ�ͬԪ����ɵĴ�����ɵ�����������10�����ӣ�E������H+��ϳ�����������EΪOH-��
�ʴ�Ϊ��F��Na��H��N��OH-��
��2��C��ͬλ��C2 Ϊ2H������Ϊ��뮣��ʴ�Ϊ��뮣�
��3��C��D�γɷ���ΪNH3������ʽΪ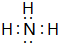 ���ռ�ṹΪ�������Σ��ʴ�Ϊ��
���ռ�ṹΪ�������Σ��ʴ�Ϊ��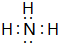 �������Σ�
��������
���� ���⿼��Ԫ�ػ������ƶϣ���Ҫѧ����������Ԫ�ػ��������ʣ��ѶȲ���

��1.5mol H2 ������ԭ�ӵ����ʵ���Ϊ4mol��H2 ��45g H2O ��16g O2��
| A�� | �٢ڢۢ� | B�� | �ۢڢ٢� | C�� | �ڢۢ٢� | D�� | �ڢ٢ۢ� |
| A�� | ��״���£�2.24L CCl4������ĿΪ0.1NA | |
| B�� | ���ʵ���Ũ��Ϊ0.25mol/L��MgCl2��Һ�У�����Cl-����Ϊ0.5NA | |
| C�� | 6.4g O2��O3�Ļ�����к��е���ԭ����ĿΪ0.4NA | |
| D�� | �ú�3.01��1023��FeCl3�ı�����Һ�Ʊ�������������������ĿΪ0.5NA |
��C2H2��C2H4O ��C4H8��C6H12O6 ��C7H8��C6H12 ��HCOOCH3��CH3COOH��
| A�� | �٢ۢ� | B�� | �٢ڢۢ� | C�� | �٢� | D�� | �٢ڢ� |
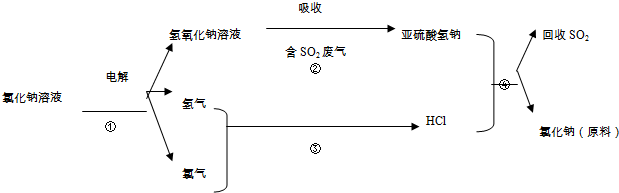
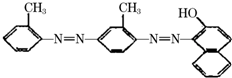 ���������̨ÿ���������汨���������г������۵�һ���ֱ���ǰ�����ز��ġ�����Ѽ������ż��Ⱦ�ϡ��յ�����š������ʰ�֢�о�����������Ϊ�����°�����յ�����š��Ľṹ��ʽ��ͼ�����й��ڡ��յ�����š�˵����ȷ���ǣ�������
���������̨ÿ���������汨���������г������۵�һ���ֱ���ǰ�����ز��ġ�����Ѽ������ż��Ⱦ�ϡ��յ�����š������ʰ�֢�о�����������Ϊ�����°�����յ�����š��Ľṹ��ʽ��ͼ�����й��ڡ��յ�����š�˵����ȷ���ǣ�������| A�� | ���ڷ����� | B�� | ���ڱ���ͬϵ�� | C�� | ���ڰ����� | D�� | �ܷ����ӳɷ�Ӧ |
| A�� | ����ӡˢ��·�壺Fe3++Cu�TFe2++Cu2+ | |
| B�� | ��ʯī�����缫���CuCl2��Һ��Cu2++2Cl-$\frac{\underline{\;ͨ��\;}}{\;}$ Cu+Cl2�� | |
| C�� | ��������Һ���������������Һ��Ӧ�����ӷ���ʽ��Al3++3OH-�TAl��OH��3�� | |
| D�� | �ô����ɳ�ˮ���ķ�Ӧ��CaC03+2H+�TCa2++C02��+H20 |
| A�� | 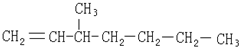 | B�� | 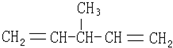 | ||
| C�� | 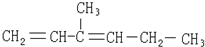 | D�� | 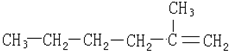 |