��Ŀ����
���з�Ӧһ�����ڷ��Է����е���
| A��N2(g) + 2O2(g) = 2NO2(g) ��H =" +" 67.7 kJ/mol |
| B��CaCO3(s) ��CaO(s) ��CO2(g) ��H =" +" 1921.8 kJ/mol |
| C��C6H12O6 ( s ) + 6O2 ( g ) = 6CO2 (g) + 6H2O ( l )��H =" -2804" kJ/mol |
| D��2CO(g)+O2 (g)=2CO2(g)��H= ��566 kJ/mol |
A
�ɡ�G����H��T��S��֪��H>0�ҡ�S<0ʱ��һ��Ϊ���Է����з�Ӧ��A��������⣨����ϵ����С����S<0��

��ϰ��ϵ�д�
�����Ŀ
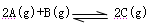 ����H<0�����и�ͼ��ȷ���ǣ���仯���������ʵľۼ�״̬û�з����ı䣩
����H<0�����и�ͼ��ȷ���ǣ���仯���������ʵľۼ�״̬û�з����ı䣩 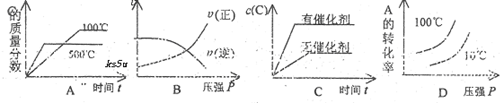
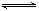 2C(g)���ڲ�ͬ�¶��¾���һ��ʱ�䣬�������C������������¶ȵĹ�ϵ��ͼ��ʾ����д���пհף�
2C(g)���ڲ�ͬ�¶��¾���һ��ʱ�䣬�������C������������¶ȵĹ�ϵ��ͼ��ʾ����д���пհף�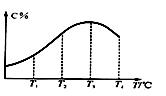
 ��g��
��g�� CO��g����3H2��g����H��0���ﵽƽ��״̬������������ȷ���� �� ��
CO��g����3H2��g����H��0���ﵽƽ��״̬������������ȷ���� �� ��