��Ŀ����
��12�֣���1����֪��25��ʱ�����ᡢ̼���������ĵ���ƽ�ⳣ���ֱ�Ϊ��
���� Ki = 1.75��10��5
̼�� Ki 1= 4.30��10��7 Ki 2 = 5.61��10��11
������ Ki 1= 1.54��10��2 Ki 2 = 1.02��10��7
д��̼��ĵ�һ������ƽ�ⳣ������ʽ��Ki = _ __
����ͬ�����£��ԱȽ�H2CO3��HCO3����HSO3����������ǿ����
�� �������¶Ȳ��䣬�ڴ�����Һ�м����������ᣬ���������С���� ������ţ�
A. c(CH3COO��) B. c(H+) C. w�������ƽ�ⳣ�� D. ����ĵ����  ��2��һ���¶��µ����ܵ����AmBn��ˮ��Һ�дﵽ�����ܽ�ƽ��ʱ����ƽ�ⳣ��
��2��һ���¶��µ����ܵ����AmBn��ˮ��Һ�дﵽ�����ܽ�ƽ��ʱ����ƽ�ⳣ��
Ksp��cm(An��)��cn(Bm��)����Ϊ���ܵ���ʵ����ӻ�����25��ʱ��AgCl�İ�ɫ����Һ�У����μ����Ũ�ȵ�KI��Һ��Na2S��Һ���۲쵽���������ȳ��ֻ�ɫ������������ɺ�ɫ������
������������ȷ����  w.w.w.zxxk.c.o.m
w.w.w.zxxk.c.o.m
A���ܶȻ�С�ij�������ת��Ϊ�ܶȻ���С�ij���
B�����ȼ���Na2S��Һ���ټ���KCl��Һ�����ް�ɫ��������
C��25��ʱ������AgCl��AgI��Ag2S��Һ������Ag����Ũ����ͬ
D��25��ʱ��AgCl�����ڵ����ʵ���Ũ�ȵ�NaCl��CaCl2��Һ�е��ܶȻ���ͬ
��3�������£�ȡpH=2������ʹ�����Һ��100mL�������зֱ����������Zn������Ӧ����������Һ��pH�仯��ͼ��ʾ����ͼ�б�ʾ������Һ��pH�仯���ߵ��� ���A����B������
�������м����Zn����Ϊm1�� ������Һ�м����Zn����Ϊm2����m1 m2��ѡ���������=������������
��1����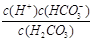 �� H2CO3 �� A D ��2��C
�� H2CO3 �� A D ��2��C
��3��B��<
���������������1����д��̼���һ������ĵ��뷽��ʽ�����ݵ���ƽ�ⳣ���ı���ʽ��Ki���ڵ���ƽ�ⳣ��Խ������Խǿ��̼������Կ���һ�������ƽ�ⳣ����HSO3�������Կ�������ĵڶ��������ƽ�ⳣ��Ki2���ۼ������ᣬ������Ũ��������ĵ���ƽ�������ƶ�������̶ȼ�С����������ӵ�Ũ�ȼ�С�������ӵ�Ũ��������ƽ�ⳣ��ֻ���¶��йأ��ʵ���ƽ�ⳣ�����䣬��ѡAD����2��A�����ܵĿ���ת���ɸ����ܵģ���A��ȷ��B�����������������������Ȼ������ܽ������ʲ���ת�����Ȼ�����������B��ȷ�������ߵ�Ksp����ͬ�����ܽ���������ͬ�������ߵı�����Һ�е������ӵ�Ũ�Ȳ���ͬ����C����D���ܶȻ�ֻ���¶��йأ�����������Һ���أ�����������Һ�е��ܶȻ���ͬ����D��ȷ����ѡC����3����ʼʱ����Һ�е�������Ũ����ȣ�������Һ�л����ڴ���ĵ���ƽ�⣬���ŷ�Ӧ�Ľ��У������ӵ����ģ�����ĵ���ƽ�������ƶ���ʹ�����ӵ�Ũ�����ʴ�����Һ�е�������Ũ�ȼ�С��������B�����Ǵ����pH��ȣ�����ʼ������Ũ����ȵ������£���������ʵ���Ũ�ȴ��������ȵ������£��������ĵ�п�ࡣ
���㣺����ƽ�ⳣ���ı���ʽ������ƽ���Ӱ�����ء��ܽ�ƽ���Ӱ�����ء��ܶȻ���Ӱ�����ص�֪ʶ��

 ������ÿ�ʱ��ҵϵ�д�
������ÿ�ʱ��ҵϵ�д������£�ijŨ�ȵĴ�����Һ��n(CH3COO��)=0.01mol������������ȷ����
| A����ô�����Һ�м���������Ũ�ȵ����ᣬ���ƴ���ĵ��룬��Һ��c(H+)���� |
| B����������Һ���Ϊ1L����c(CH3COOH)=0.01mol/L |
| C����NaOHǡ���к�ʱ����Һ��c(Na+)��c(CH3COO��) |
| D����������Ũ�ȵĴ�������Һ��ϣ���Һ��c(Na+)+ c(H+)=c(CH3COO��)+ c(OH��) |
����˵���д������
| A��0.5 L 2 mol��L��1 AlCl3��Һ�У�Al3����Cl������С��4��6.02��1023�� |
| B���Ƴ�0.5 L 10 mol��L��1�����ᣬ��Ҫ��״���µ��Ȼ�������112 L |
| C����1 L 1 mol��L��1��NaCl��Һ����ȡ��10 mL��Һ����Ũ����1 mol��L��1 |
| D��10 g 98%������(�ܶ�Ϊ1.84 g��cm��3)��10 mL18.4 mol��L��1�������Ũ�Ȳ�ͬ |
��1�������¶���a mL pH="13" NaOH��Һ��b mL0.05mol/L H2SO4��ϣ����û����Һ��pH=7��
��a:b =
��2�����ں�������AgCl����ı���AgCl��Һ�зֱ���룺
| A��100mL����ˮ�У� | B��100mL 0.2 mol��L��1AgNO3��Һ�У� |
| C��100 mL 0.1 mol��L��1AlCl3��Һ�У� | D��100mL 0.1 mol��L��1������Һ�С� |
��3����25���£���Ũ�Ⱦ�Ϊ0.20 mol��L-1��MgCl2��CuCl2�����Һ����μ��백ˮ�������� �������ѧʽ�������ɸó��������ӷ���ʽΪ ���������ҺpH=11.00ʱ������¶��²�������Һ�е�c(Mg2+):c(Cu2+)=
����֪25��ʱKsp[Mg(OH)2]=1.8��10-11��KsP[Cu(OH)2]=2.0��10-20��
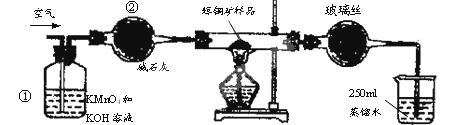
��15�֣���ҵ��Ϊ�˲ⶨ��ͭ����Ҫ�ɷ���Cu2S����Cu2S�������������������ͼװ�á�ʵ��ʱ�����²��������
| A�����Ӻ�������ʹ���Ϊ��ͼװ�ã������װ�õ������ԡ� |
| B����ȡ��ϸ�Ļ�ͭ����Ʒ1.000g�� |
| C���������õ���ƷС�ĵط���Ӳ�ʲ������С� |
| D����ÿ����1L�����ʹ�������� |
 SO2 +2Cu��
SO2 +2Cu��F.��ȡ25.00mL��SO2��ˮ��Һ��250mL��ƿ�У���0.0100mol/L KMnO4����Һ�ζ����յ㡣���������������ظ��ζ�2��3�Ρ�
�Իش��������⣺
��1��װ�âٵ�������_________________��װ�âڵ�������____________________��
��2���ٶ���ͭ���е���ȫ��ת��ΪSO2������ȫ����ˮ���գ������F����������Ӧ�Ļ�ѧ����ʽΪ ��������_______________________________������ʱ���жϵζ��Ѿ��ﵽ�յ㡣
��3��������F�ĵζ�������±���ʾ�����ͭ����Ʒ��Cu2S������������________��
| �ζ� ���� | ������Һ�� ���/mL | ����Һ����� | |
| �ζ�ǰ�̶�/mL | �ζ���̶�/mL | ||
| 1 | 25.00 | 1.04 | 21.03 |
| 2 | 25.00 | 1.98 | 21.99 |
| 3 | 25.00 | 3.20 | 21.24 |
��5����֪�ڳ�����FeS �� Ksp�� 6 . 25 �� 10 ��18, H2S ������Һ�� c (H������ c (S2����֮��������¹�ϵ��c2 (H��)����S2��) =" 1" . 0��10��22���ڸ��¶��£������� FeS Ͷ�����ⱥ����Һ�У���ʹ��Һ�У�Fe2+��Ϊ1 mol/L��Ӧ������Һ��c��Hʮ��Ϊ__________________��

