��Ŀ����
����Ŀ������������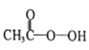 )��һ�ֳ�������������ǿ������,��ѹ�������Ʊ����������������װ�����£�
)��һ�ֳ�������������ǿ������,��ѹ�������Ʊ����������������װ�����£�
����������ƿ�м���һ������������ŨH2SO4�����Һ������������������30%��˫��ˮ��
�ڲ��Ͻ��貢����B�л��Һ���¶�Ϊ20��30��һ��ʱ�䡣
�۽��������ܺͳ����ã�����ƿ���ռ��õ���Ʒ��

��ͬ��Ӧ�������ʵ�������ɹ������Ậ��(%)��ʱ��ı仯����(���±�)����ش�������⣺
��Ӧ����� CH3COOH/H2O2 | ��Ӧʱ��(Сʱ) | ||||
0.5 | 1 | 3 | 5 | 7 | |
2:1 | 7.38 | 8.46 | 9.42 | 11.26 | 13.48 |
1:1 | 10.56 | 12.92 | 13.54 | 20.72 | 20.70 |
1:2 | 6.14 | 7.10 | 7.96 | 10.38 | 12.36 |
(1)C��������ˮ�������_____(��a��b)�����ɹ�������Ļ�ѧ����ʽ��__________��
(2)��Ӧ����ѱ���(CH3COOH/H2O2)��______����Ӧ����ʱ��Լ________(ѡ��1��3��5��7)Сʱ��
(3)Ϊ���ÿ��Ʒ�Ӧ�¶ȣ�Ӧ���ӵĴ�ʩ��________��
(4)�����ʵ��Ƚ�Fe3+��Cu2+�Թ�������Ĵ�Ч�ʡ�
�ɹ�ѡ����Լ��������У�a.����������Һ��b.1mol/L��FeCl3��Һ��c.0.5mol/L��Fe2(SO4)3��Һ��d. 0.5mol/L��CuCl2��Һ��e.1mol/L��CuSO4��Һ��f.��ʱ����g.���������������Ͳ��
��ѡ����Լ���������a��________��f��g(ѡ����ţ�ʵ�鷽����____________��
(5)��Ʒ�����ⶨ��
ȡ2.00mL������������ϡ�ͳ�100mL������ȡ��5.00mL���μ�����KMnO4��Һ��ǡ�÷ۺ�ɫ���Գ�ȥ����H2O2������10mL10%KI��Һ�ͼ��ε�����Һ��ҡ�ȣ���0.1000mol/L��Na2S2O3��Һ�ﵽ���յ�(��Ӧ����ʽΪ2Na2S2O3+I2=Na2S4O6+2NaI)��������13.20mL��Na2S2O3��Һ������Ʒ�й�����������ʵ���Ũ����____________��
���𰸡� a CH3COOH+ H2O2 ![]() CH3COOOH+H2O 1��1 5 B�ô��¶ȼƵ�ˮԡ���� c e �Թ�������Ͳ�����װ�������ԣ�ȡ�����Ĺ���������Һ����a�������Թ��У��ٷֱ���������0.5 mol/L��Fe2(SO4)3��Һ����c����Imol/L��CuSO4��Һ����e������¼��ͬʱ�����������������������������ʱ�䣩�� 6.6mol/L
CH3COOOH+H2O 1��1 5 B�ô��¶ȼƵ�ˮԡ���� c e �Թ�������Ͳ�����װ�������ԣ�ȡ�����Ĺ���������Һ����a�������Թ��У��ٷֱ���������0.5 mol/L��Fe2(SO4)3��Һ����c����Imol/L��CuSO4��Һ����e������¼��ͬʱ�����������������������������ʱ�䣩�� 6.6mol/L
��������(1)C�����������ܣ���ˮ������ѭ�½��ϳ����������a�������ᱻ˫��ˮ�������ɹ������ᣬ��Ӧ�Ļ�ѧ����ʽΪCH3COOH+ H2O2![]() CH3COOOH+H2O���ʴ�Ϊ��a��CH3COOH+ H2O2
CH3COOOH+H2O���ʴ�Ϊ��a��CH3COOH+ H2O2![]() CH3COOOH+H2O��
CH3COOOH+H2O��
(2)���ݱ������ݿ�֪����Ӧ����ѱ���(CH3COOH/H2O2)��1:1����Ӧ����ʱ��Լ5Сʱ���ʴ�Ϊ��1��1 �� 5��
(3)Ϊ���ÿ��Ʒ�Ӧ�¶ȣ�BӦ���ô��¶ȼƵ�ˮԡ���£��ʴ�Ϊ��B�ô��¶ȼƵ�ˮԡ���£�
(4)����ʵ��Ŀ�ģ��Ƚ�Fe3+��Cu2+�Թ�������Ĵ�Ч�ʡ����������ӿ��ܱ���������������Ӱ��ʵ�������жϣ���Ҫѡ����Լ���������a.����������Һ�� c.0.5mol/L��Fe2(SO4)3��Һ�� e.1mol/L��CuSO4��Һ��f.��ʱ����g.���������������Ͳ������ʵ�鷽��Ϊ�Թ�������Ͳ�����װ�������ԣ�ȡ�����Ĺ���������Һ(��a)�����Թ��У��ٷֱ���������0.5 mol/L��Fe2(SO4)3��Һ(��c)��Imol/L��CuSO4��Һ(��e)����¼��ͬʱ������������(���������������ʱ��)���ʴ�Ϊ��c e���Թ�������Ͳ�����װ�������ԣ�ȡ�����Ĺ���������Һ(��a)�����Թ��У��ٷֱ���������0.5 mol/L��Fe2(SO4)3��Һ(��c)��Imol/L��CuSO4��Һ(��e)����¼��ͬʱ������������(���������������ʱ��)��
(5) 0.1000mol/L��Na2S2O3��Һ13.20mL�к���Na2S2O3�����ʵ���Ϊ0.00132mol�����ݷ���ʽ2Na2S2O3+I2=Na2S4O6+2NaI����Ӧ�ĵ�Ϊ0.00066mol��ת�Ƶ���0.00132mol�����ݵ�ʧ�����غ㣬������������ʵ���Ϊ0.00066mol��Ũ��Ϊ = 6.6mol/L���ʴ�Ϊ��6.6mol/L��
= 6.6mol/L���ʴ�Ϊ��6.6mol/L��

 ���Ͱ�ͨ�������Сѧ��ʱͬ�����ϵ�д�
���Ͱ�ͨ�������Сѧ��ʱͬ�����ϵ�д� ���Ͱ�ͨ������ϵ�д�
���Ͱ�ͨ������ϵ�д�����Ŀ���±����и��������У�����֮��ͨ��һ����Ӧ����ʵ������ͼ��ʾת������
ѡ�� | X | Y | Z |
A | Na | NaOH | NaCl |
B | Si | SiO2 | Na2SiO3 |
C | Cl2 | HClO | NaClO |
D | NO | NO2 | HNO3 |

A. A B. B C. C D. D

