��Ŀ����
�����������(BN)n��һ�����ϳɲ��ϣ����ʽΪ(BN)�ݣ����и�Ӳ�ȡ����µ��ص㣬���������������մɲ��ϡ�ĥ�ϡ���ĥ�оߵĺò��ϡ�����ɰ��Na2B4O7���������ڸ��¸�ѹ�·�Ӧ���Ի�á����磺 Na2B4O7 + 2CO(NH2)2 �� 4(BN) + Na2O + 2CO2
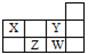
��1���������������ʽ��ʾ��ɰ�Ļ�ѧʽ ������(BN)n�Ⱦ������и���Ӳ�Ⱥ������Ե�ԭ���ǣ� ��
��21��������Ӧʽ�о���4�ֲ�ͬ�������ӵ�ԭ�ӣ���������Ԫ�������ڱ��д��ڵ� ���ڣ��� �塣
��3�������ڱȽ�N��O�ǽ��������ǿ������ʵ�� ��
��4��д���������к����Լ��ķǼ��Է��ӵĵ���ʽΪ ��
��5������ͬ�������������ڵ�Ԫ�أ��������������NaOH��Һ��Ӧ�����ӷ���ʽ Ϊ�� ��
��1���������������ʽ��ʾ��ɰ�Ļ�ѧʽ ������(BN)n�Ⱦ������и���Ӳ�Ⱥ������Ե�ԭ���ǣ� ��
��21��������Ӧʽ�о���4�ֲ�ͬ�������ӵ�ԭ�ӣ���������Ԫ�������ڱ��д��ڵ� ���ڣ��� �塣
��3�������ڱȽ�N��O�ǽ��������ǿ������ʵ�� ��
| A������������Ӧˮ��������� | B��H2O(g) ��NH3(g)�ȶ� |
| C��������H2��Ӧ�����׳̶� | D��NO�е�Ԫ�������ۣ���Ԫ���Ը��� |
��5������ͬ�������������ڵ�Ԫ�أ��������������NaOH��Һ��Ӧ�����ӷ���ʽ Ϊ�� ��
��1��Na2O��2B2O3 ������ԭ�Ӿ��壬B-N������Si-Si���̣���B-N���ܽϴ�
��2���������ڣ��ڢ�A ��
��3��D
��4��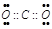
��5��Al2O3+2OH-+��2AlO2-+H2O
��2���������ڣ��ڢ�A ��
��3��D
��4��
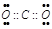
��5��Al2O3+2OH-+��2AlO2-+H2O
�����������1�����߶�����ԭ�Ӿ��壬һ����˵��ԭ�Ӱ뾶ԽС���γɹ��ۼ��ļ���Խ�̣�����Խ���侧���۷е�Խ�ߡ�B-N������Si-Si���̣�����(BN)n�Ⱦ������и���Ӳ�Ⱥ������ԡ�
��2������4�ֲ�ͬ�������ӵ�ԭ��Ϊ��ԭ�ӣ������Ų�ʽΪ1S22S22P63S1,��Ϊ�������ڣ��ڢ�A �塣
��3���ǽ�����ǿ���ȽϿ��������¼��㣺1��ͬ�����У������ң���˵���������ӣ��ǽ�������ǿ��ͬ�����У����ϵ��£���˵���������ӣ��ǽ����Լ�����2����������������ˮ�������Ե�ǿ����������ǿ����Ԫ�صķǽ�����Ҳ��ǿ��3����������̬�⻯����ȶ��ԣ��ȶ�����ǿ���ǽ�������ǿ��4�����������ϵ�������5��������Һ֮����û���Ӧ��Aѡ�����OԪ��û�к����ᣬ��A����Bѡ����ȷ��Cѡ�������H2��Ӧ�����׳̶ȣ�˵�����ʵ�������ǿ�������ʵĽṹ��ͬ������˵��Ԫ�صķǽ����ԣ��ʴ���Dѡ����ϼ�˵�����õ��Ӷ�ƫ�Ʒ��������б��ָ��۵�Ԫ�أ��Լ��ϵ��ӵ���������ǿ���ǽ����Ը�ǿ��NԪ����������������ǿ���ʷǽ�����N��O�����ԱȽϡ�D��ȷ��
��4���������к����Լ��ķǼ��Է���Ϊ������̼���������ʽΪ��
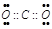
��5������ͬ�������������ڵ�Ԫ��ֻ��AlԪ�ء��ʣ���NaOH��Һ��Ӧ�����ӷ���ʽΪ��Al2O3+2OH-+��2AlO2-+H2O

��ϰ��ϵ�д�
�����Ŀ
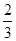 e��d��˴�����Ϊ-
e��d��˴�����Ϊ-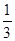 e��eΪ��Ԫ��ɣ������۶��п�����ȷ����( )
e��eΪ��Ԫ��ɣ������۶��п�����ȷ����( )