��Ŀ����
ij�о�С��ⶨ�����в��ἰ�����κ�������C2O42���ƣ���ʵ�鲽�����£�
�ٽ������� ƷԤ�������õ����в��ἰ�����ε���Һ��
ƷԤ�������õ����в��ἰ�����ε���Һ��
�ڵ�����Һ�������ԣ��μ�����CaCl2��Һ��������ɫ���������˵õ�CaC2O4���塣
����ϡHCl�ܽ�CaC2O4���100mL��Һ��Ȼ����KMnO4����Һ�ζ�2��3�Ρ�
��1��������С�����ƷԤ�������ķ����� ��
A�����ճɻҡ����ݡ����ˡ��� B����ĥե֭�����ݡ�����
��2��������С�������Һ�������ԡ���Ŀ����______________________ ��
��3������ڽ�����Ӧ��ʱ��֤CaCl2��Һ�ѡ������������������������ǣ� _��
��4����������õ��IJ����������ձ�����ƿ����ͷ�ιܡ��������⣬������ ������
��5����ʵ���еζ�����Ҫ����ָʾ�����ζ��յ��������______________________��
�ٽ�������
 ƷԤ�������õ����в��ἰ�����ε���Һ��
ƷԤ�������õ����в��ἰ�����ε���Һ���ڵ�����Һ�������ԣ��μ�����CaCl2��Һ��������ɫ���������˵õ�CaC2O4���塣
����ϡHCl�ܽ�CaC2O4���100mL��Һ��Ȼ����KMnO4����Һ�ζ�2��3�Ρ�
��1��������С�����ƷԤ�������ķ����� ��
A�����ճɻҡ����ݡ����ˡ��� B����ĥե֭�����ݡ�����
��2��������С�������Һ�������ԡ���Ŀ����______________________ ��
��3������ڽ�����Ӧ��ʱ��֤CaCl2��Һ�ѡ������������������������ǣ� _��
��4����������õ��IJ����������ձ�����ƿ����ͷ�ιܡ��������⣬������ ������
��5����ʵ���еζ�����Ҫ����ָʾ�����ζ��յ��������______________________��
��1��B����3�֣�
��2����H2C2O4ת��ΪC2O42����������ת��Ϊ���� ��3�֣�
��3�����ã����ϲ���Һ���������μ�CaCl2��Һ�����ٳ��ֻ���˵��CaCl2��������3�֣�
��4����ʽ�ζ��ܡ�����ƿ��4�֣�
��5����Һ����ɫ���dz��ɫ�Ұ�����ڲ���ɫ
��2����H2C2O4ת��ΪC2O42����������ת��Ϊ���� ��3�֣�
��3�����ã����ϲ���Һ���������μ�CaCl2��Һ�����ٳ��ֻ���˵��CaCl2��������3�֣�
��4����ʽ�ζ��ܡ�����ƿ��4�֣�
��5����Һ����ɫ���dz��ɫ�Ұ�����ڲ���ɫ
��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
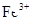

 1 mol��L��1BaCl2��0.01 mol��L��1 KMnO4������ˮ��
1 mol��L��1BaCl2��0.01 mol��L��1 KMnO4������ˮ��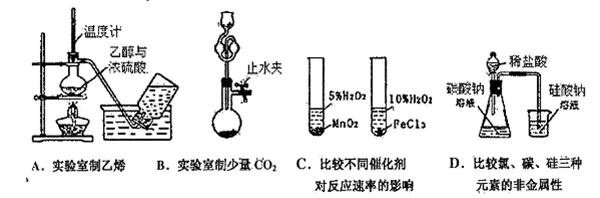
 ��֪��
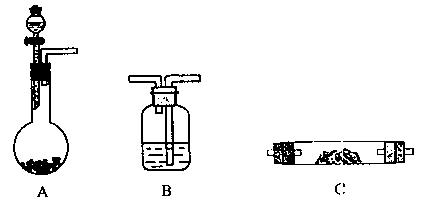
��֪��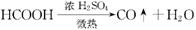 ��ʵ��������ͼ��ʾ��װ��.����ȡһ����̼��ѡ�õ�װ��Ϊ________________(����ĸ����
��ʵ��������ͼ��ʾ��װ��.����ȡһ����̼��ѡ�õ�װ��Ϊ________________(����ĸ����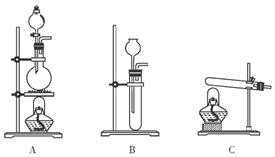

 ������ƽ��ҩ�ס����������___ _____�����������ƣ���
������ƽ��ҩ�ס����������___ _____�����������ƣ���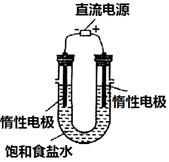
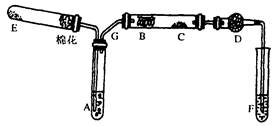
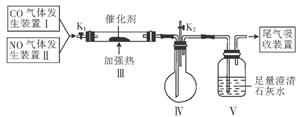
 MnCl2+Cl2��+2H2O���ݴˣ���������������װ����ѡ���Ʊ����ռ�H2��װ��_______(�����)���Ʊ����ռ��������Cl2��װ��_________������ţ���
MnCl2+Cl2��+2H2O���ݴˣ���������������װ����ѡ���Ʊ����ռ�H2��װ��_______(�����)���Ʊ����ռ��������Cl2��װ��_________������ţ���