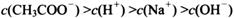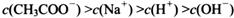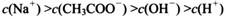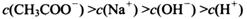��Ŀ����
��15�֣�ijʵ��С������֤Mg�ۺ�CO2�ķ�Ӧ�������ͼ��ѡ����������������ظ�ѡ�ã����һ���и�ʵ���װ�á����ṩŨH2SO4��ϡ���ᡢþ�ۡ�����ʯ������ʯ��ˮ������NaHCO3��Һ������Na2CO3��Һ�������Ӻ̶������õIJ����ܡ����ܡ����С�����̨������װ�õȾ���ȥ����
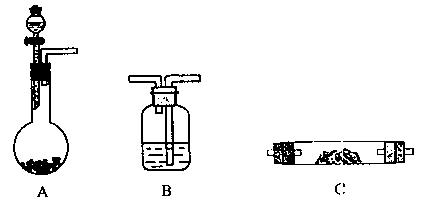
��1������ѡ������������˳�������������������±�����д����������Ӧ�ӵ��Լ����ɲ�������
��2��A�з�����Ӧ�����ӷ���ʽΪ ��
��3����װ��������������������ã�����ҩƷ���ڼ��ȷ�Ӧ��C֮ǰӦ���еIJ�����Ŀ����
��
��4����Ӧ��Cװ�õ���Ҫ������ ��

��1������ѡ������������˳�������������������±�����д����������Ӧ�ӵ��Լ����ɲ�������
| ѡ�õ�����������ĸ�� | ������Լ� |
| A | ϡ���ᡢ����ʯ |
| | |
| | |
| | |
| | |
��3����װ��������������������ã�����ҩƷ���ڼ��ȷ�Ӧ��C֮ǰӦ���еIJ�����Ŀ����
��
��4����Ӧ��Cװ�õ���Ҫ������ ��
��15�֣�

��

��ϰ��ϵ�д�
�����Ŀ
 ƷԤ�������õ����в��ἰ�����ε���Һ��
ƷԤ�������õ����в��ἰ�����ε���Һ��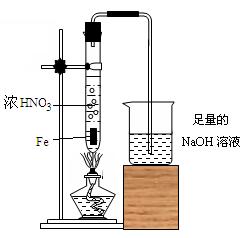
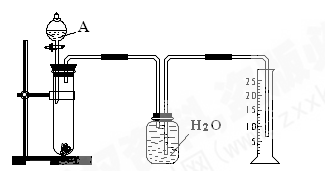
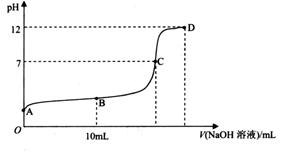
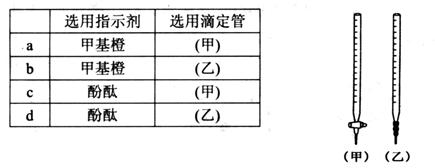
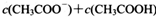 ________
________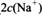 (ѡ�>������<����=��)��
(ѡ�>������<����=��)��