��Ŀ����
ij��ѧ��ȤС���ú���������ͭ�ĺϽ������ȡ�������Ȼ�����Һ���̷����壨FeSO4??7H2O���͵������壨CuSO4??5H2O�����������£�
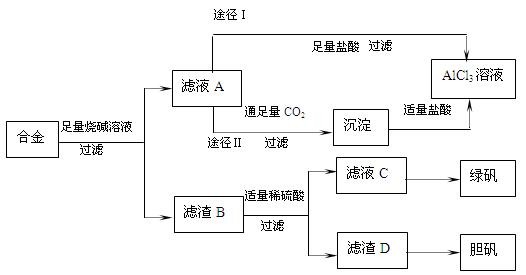
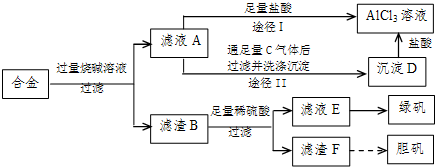
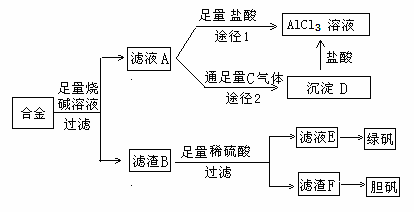
��1��д���Ͻ����������ռ���Һʱ��ط�Ӧ�����ӷ���ʽ ��
��2������ҺC�еõ��̷���ʵ�����������Ũ���� �����ˡ�ϴ�ӵȡ�
��3��H2O2��һ��Ӧ�ù㷺����ɫ��������������D�м���ϡ�����H2O2�����Ʊ��������壬�÷�Ӧ���ܻ�ѧ����ʽΪ ��
��4������ҺAͨ��;��I��;��II���ɵõ�AlCl3��Һ���Ӳ�Ʒ���ȽǶȿ��ǣ�����Ϊ��������� ���;��I����;��II������������ ��
��5���������������õ�ϡ����������������Ϊ36.8%����ô100mL98%��Ũ���ᣨ�ܶ�Ϊ1.84g/mL�������Ƴ�����ϡ�������� ����g��
��16�֣�
��1��2Al��2OH����2H2O��2AlO2��+3H2����3�֣���
��2����ȴ�ᾧ��2�֣�
��3��Cu��H2O2��H2SO4��3H2O��CuSO4��5H2O����Cu��H2O2��H2SO4��CuSO4��2H2O������3�֣�
��4��;����2�֣���;��I�Ƶõ�AlCl3��Һ�к��д�����NaCl����;�������Ƶô�����AlCl3��Һ����3�֣�
��5��490��3�֣�