��Ŀ����
14��W��M��X��Y��Z�����ڱ�ǰ36��Ԫ���е����ֳ���Ԫ�أ���ԭ��������������W��һ�ֺ����ڿ���ʱ����������һЩ����������M���������ǵ����������Ҫ����֮һ��X��ijһ�ֵ����ڸ߿մ������б�����������̫������������ǿ����Ϯ��Y�Ļ�̬ԭ�Ӻ�����6��ԭ�ӹ�����ڰ����״̬��Z���γɺ�ɫ��Z2O�ͺ�ɫ��ZO�����������1��Y3+��̬�ĵ����Ų�ʽ�ɱ�ʾΪ1s22s22p63s23p63d3��
��2��MX3-�Ŀռ乹��ƽ�������Σ���������������
��3��M���γɶ����⻯�����MH3�ļ���ǿ��M2H4��ԭ����N2H4�з���NΪ-2�ۣ���NH3������NΪ-3�ۣ������Ը��ߣ�����Nԭ�ӵŶԵ��Ӹ����ṩ������H+��ϣ��ʼ��Ը�ǿ��
��4�����ݵȵ���ԭ����WX���ӵĽṹʽΪC��O��
��5��1mol WX2�к��еĦҼ���ĿΪ2NA��
��6��H2X������Xԭ�ӹ�����ӻ�����Ϊsp3��
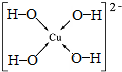
��7����Z2+����Һ�м������NaOH��Һ��������Z����λ��Ϊ4����λ���ӣ�д������λ���ӵĽṹʽ
 ��
��
���� W��M��X��Y��Z�����ڱ�ǰ36��Ԫ���е����ֳ���Ԫ�أ���ԭ��������������W��һ�ֺ����ڿ���ʱ����������һЩ������������WΪCԪ�أ�X��ijһ�ֵ����ڸ߿մ������б�����������̫������������ǿ����Ϯ����XΪOԪ�أ�M���������ǵ����������Ҫ����֮һ��ԭ������С��O����MΪNԪ�أ�Z���γɺ�ɫ��Z2O�ͺ�ɫ��ZO�����������ZΪCu��Y�Ļ�̬ԭ�Ӻ�����6��ԭ�ӹ�����ڰ����״̬��ԭ������С��Cu�����������Ų�ʽΪ1s22s22p63s23p63d54s1����YΪCrԪ�أ��ݴ˽��
��� �⣺W��M��X��Y��Z�����ڱ�ǰ36��Ԫ���е����ֳ���Ԫ�أ���ԭ��������������W��һ�ֺ����ڿ���ʱ����������һЩ������������WΪCԪ�أ�X��ijһ�ֵ����ڸ߿մ������б�����������̫������������ǿ����Ϯ����XΪOԪ�أ�M���������ǵ����������Ҫ����֮һ��ԭ������С��O����MΪNԪ�أ�Z���γɺ�ɫ��Z2O�ͺ�ɫ��ZO�����������ZΪCu��Y�Ļ�̬ԭ�Ӻ�����6��ԭ�ӹ�����ڰ����״̬��ԭ������С��Cu�����������Ų�ʽΪ1s22s22p63s23p63d54s1����YΪCrԪ�أ�
��1��Cr3+��̬�ĵ����Ų�ʽΪ1s22s22p63s23p63d3���ʴ�Ϊ��1s22s22p63s23p63d3��
��2��NO3-��Nԭ�Ӽ۲���Ӷ���=3+$\frac{5+1-2��3}{2}$=3��Nԭ��û�й¶Ե��ӣ�����ռ乹��Ϊƽ�������Σ�
�ʴ�Ϊ��ƽ�������Σ�
��3��N2H4�з���NΪ-2�ۣ���NH3������NΪ-3�ۣ������Ը��ߣ�����Nԭ�ӵŶԵ��Ӹ����ṩ������H+��ϣ��ʼ��Ը�ǿ��
�ʴ�Ϊ��N2H4�з���NΪ-2�ۣ���NH3������NΪ-3�ۣ������Ը��ߣ�����Nԭ�ӵŶԵ��Ӹ����ṩ������H+��ϣ��ʼ��Ը�ǿ��
��4��CO��N2��Ϊ�ȵ����壬���ݵȵ���ԭ�������߽ṹ���ƣ���CO�ĽṹʽΪC��O��
�ʴ�Ϊ��C��O��
��5��CO2�ĽṹʽΪO=C=O��1mol CO2�к��е�2mol�Ҽ����ʺ��ЦҼ���ĿΪ2NA��
�ʴ�Ϊ��2NA��
��6��H2O������Oԭ�ӳ�2���Ҽ�������2�Թ¶Ե��ӣ����ӻ������ĿΪ4��Oԭ�Ӳ�ȡsp3�ӻ���
�ʴ�Ϊ��sp3��
��7����Cu2+����Һ�м������NaOH��Һ��������Cu����λ��Ϊ4����λ���ӣ�����λ���ӵĽṹʽΪ�� ��
��
�ʴ�Ϊ�� ��
��
���� �����Ƕ����ʽṹ�Ŀ��飬�ƶ�Ԫ���ǽ���ؼ����漰��������Ų������ӹ��͡��������ʡ��ȵ����塢�ӻ��������ѧ���������ȣ���Ҫѧ��ȫ�����ջ���֪ʶ���Ѷ��еȣ�

| A�� | ��̼��ĥ�ɷ�ĩ���Լӿ췴Ӧ���� | |
| B�� | �����¶ȿ��Լӿ췴Ӧ���� | |
| C�� | �����������ʱ�������г���N2����Ӧ���ʲ��� | |
| D�� | ����̼���������Լӿ췴Ӧ���� |
| A�� | CH2=CH2+Br2��CH2BrCH2Br���ӳɷ�Ӧ | |
| B�� | 2CH2=CH2+O2$��_{��}^{����}$2CH3CHO��ȡ����Ӧ | |
| C�� | CHCl3+HF��CHFCl2+HCl��ȡ����Ӧ | |
| D�� | CH2COOH+CH2CH2OH$?_{��}^{Ũ����}$CH3COOCH2CH2+H2O��������Ӧ |
| A�� | ����������ˮ �ƾ���ˮ | B�� | ���ͺ�ˮ ����ˮ | ||
| C�� | �����ͺ�ˮ �����ˮ | D�� | ����ˮ ������Ҵ� |
| A | B | C | D | |
| װ�� | 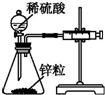 | 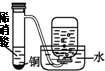 | 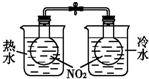 | 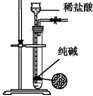 |
| ʵ�� | ���������Բ���п������ķ�Ӧ���� | �Ʊ����ռ�����NO���� | ��֤�¶ȶԻ�ѧƽ���Ӱ�� | ʵ������ʱ��ȡ����CO2���� |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | vA=0.5 mol•L-1•s-1 | B�� | v B=0.5 mol•L-1•s-1 | ||
| C�� | vC=0.4 mol•L-1•s-1 | D�� | vD=1.2 mol•L-1•min-1 |
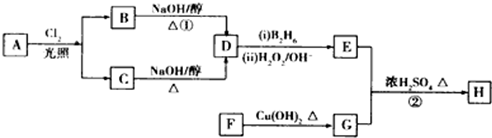
 ��
�� ��
�� ��
��