��Ŀ����
����Ŀ���о��Ϳ���CO2��CO�Ĵ��������ǻ�����������Դ����˫Ӯ�Ŀ��⡣
��1��CO�����ںϳɼ״���������ɱ���ܱ������г���4molCO��8molH2���ڴ��������ºϳɼ״���CO��g��+2H2��g��![]() CH3OH��g��(��)��ƽ��ʱCO��ת�������¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ��
CH3OH��g��(��)��ƽ��ʱCO��ת�������¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ��

���÷�Ӧ���淴Ӧ����________��Ӧ����������������������������
����0.1Mpa ��100���������£��÷�Ӧ�ﵽƽ��ʱ�������Ϊ��ʼ���������_________���������������λС���㣩
�����¶Ⱥ��ݻ����������£�����ƽ����ϵ�г���4molCO���ﵽƽ��ʱCOת����________����������������������������С������ƽ�ⳣ��K________����������������������������С������
��2���ڷ�Ӧ(��)����Ҫ�õ�H2����Ӧ��Լ���Ϊԭ����ȡ�����ǹ�ҵ�ϳ��õ����ⷽ������֪��
��CH4��g��+ H2O��g��= CO��g��+3H2��g�� ��H=+206.2 kJ��mol-1
��CH4��g��+ CO2��g��= 2CO��g��+2H2��g�� ��H=+247.4 kJ��mol-1
��CH4��H2O��g����Ӧ����CO2��H2���Ȼ�ѧ����ʽΪ�� ��
��3���ڷ�Ӧ(��)���Ƶõ�CH3OH ��������ȼ�ϣ���������������ɼ���ȼ�ϵ�أ��������Һ��20����30����KOH��Һ�����ȼ�ϵ�طŵ�ʱ�������ĵ缫��ӦʽΪ____________________��
���𰸡���1������ ��2��0.67 ��3����С ����
��4��CH4(g)+2H2O(g)��CO2(g)+4H2(g) ��H=+165.0 kJ��mol-1
��5��CH3OH+8OH--6e-=CO32-+6H2O
��������
�����������1������ͼ��֪��ѹǿһ��ʱ�����¶����ߣ�CO��ת���ʽ��ͣ��������¶�ƽ�����淴Ӧ�ƶ���������ӦΪ���ȷ�Ӧ������淴Ӧ�����ȷ�Ӧ��
��100����ƽ��ʱCO��ת����Ϊ0.5�����Բμӷ�Ӧ��CO�����ʵ���Ϊ2mol��
CO��g��+2H2��g��![]() CH3OH��g����
CH3OH��g����
��ʼ��mol���� 4 8 0
�仯��mol���� 2 4 2
ƽ�⣨mol���� 2 4 2
��˸÷�Ӧ�ﵽƽ��ʱ�������Ϊ��ʼ���������![]() ����
����
�����¶Ⱥ��ݻ����������£�����ƽ����ϵ�г���4molCO��ƽ��������Ӧ�ƶ�����CO��ת������С����ѧƽ�ⳣ��ֻ���¶�Ӱ�죬��Ũ���أ��¶Ȳ��䣬ƽ�ⳣ�����䣻
��2����CH4��g��+H2O��g��=CO��g��+3H2��g����H=+206.2kJmol-1
��CH4��g��+CO2��g��=2CO��g��+2H2��g����H=+247.4kJmol-1
���ݸ�˹���ɼ��㣬�١�2�������õ�CH4��H2O��g����Ӧ����
CO2��H2���Ȼ�ѧ����ʽΪCH4(g)+2H2O(g)��CO2(g)+4H2(g) ��H=+165.0 kJ��mol-1 ��
��3����ȼ�ϵ�طŵ�ʱ��ԭ��أ����������״�ʧ���ӷ���������ԭ��Ӧ����̼���Σ���Ӧ�ĵ缫��ӦʽΪCH3OH+8OH--6e-=CO32-+6H2O��

 ���ɶ��ȫ���ƿؾ�ϵ�д�
���ɶ��ȫ���ƿؾ�ϵ�д�����Ŀ������ˮ��ɽ���ǽ�ɽ��ɽ�����о�NO2��NO��CO��S02�ȴ�����Ⱦ���ˮ��Ⱦ��Ĵ����Խ��������й�������Ҫ���塣
(1)��֪�� ��NO2+CO![]() CO2+NO�÷�Ӧ��ƽ�ⳣ��ΪK1(��ͬ)��ÿ1mol�������ʷֽ�Ϊ��̬��̬ԭ�����յ������ֱ�Ϊ
CO2+NO�÷�Ӧ��ƽ�ⳣ��ΪK1(��ͬ)��ÿ1mol�������ʷֽ�Ϊ��̬��̬ԭ�����յ������ֱ�Ϊ
NO2 | CO | CO2 | NO |
812kJ | 1076kJ | 1490kJ | 632kJ |
��N2(g)+O2(g) ![]() 2NO(g) ��H=+179.5kJ/mol K2
2NO(g) ��H=+179.5kJ/mol K2
��2NO(g)+O2(g)![]() 2NO2(g) ��H=-112.3kJ/mol K3
2NO2(g) ��H=-112.3kJ/mol K3
д��NO��CO��Ӧ��������Ⱦ������Ȼ�ѧ����ʽ____________________________________�����Ȼ�ѧ����ʽ��ƽ�ⳣ��K=_________(��K1��K2��K3��ʾ)��
(2)������ɱ�ĺ�ѹ(p��)�ܱ������г���1molCO2 ��������̼�����䷢����Ӧ�� C(s)+ CO2(g)![]() 2CO(g) ��H>0��ƽ��ʱ����ϵ����������������¶ȵĹ�ϵ����ͼ��ʾ��
2CO(g) ��H>0��ƽ��ʱ����ϵ����������������¶ȵĹ�ϵ����ͼ��ʾ��
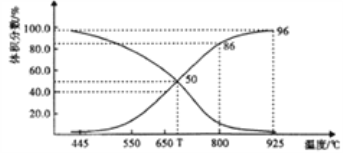
��T��ʱ����������������ϡ�����壬v(��)___v(��)(�>����<����=")��ƽ��______�ƶ�(���������������ͬ)��������������CO2 ��CO��ƽ��________�ƶ���
��CO�������Ϊ40%ʱ��CO2 ��ת����Ϊ_______��
����֪�������ѹ(p��)=������ѹ�������������ƽ���ѹ����ƽ��Ũ�ȱ�ʾƽ��Kp�����ı���ʽΪ__________��925��ʱ��Kp=______(�ú�p���Ĵ���ʽ��ʾ)��
(3)ֱ���ŷź�SO2 ���������γ����꣬Σ������������NaOH���գ����ú������(H2SO3��HSO3-��SO32-)�����ڷ�Ӧ�����Һ�У����ǵ����ʵ�������X(i)����ҺpH�Ĺ�ϵ��ͼ��ʾ��

������0.1mol/LNaOH ��Ӧ�����Һ�������Һ��pH=8ʱ����Һ�и�����Ũ���ɴ�С��˳����______________��
����pH=5��NaHSO3��Һ�еμ�һ��Ũ�ȵ�CaCl2 ��Һ����Һ�г��ֻ��ǣ�pH��Ϊ2���û�ѧƽ���ƶ�ԭ��������ҺpH���͵�ԭ��_______________________________________________��
����Ŀ�������������һ����Ҫ�Ļ�����Ʒ��ij��ȤС�����Ʊ���������ƾ���(Na2S2O3��5H2O)��
����[��������]
��1��Na2S2O3��5H2O����ɫ�����壬������ˮ����ϡ��Һ��BaCl2��Һ����������ɡ�
��2����Na2CO3��Na2S���Һ��ͨ��SO2���Ƶ�Na2S2O3�����ò�Ʒ�г���������Na2SO3��Na2SO4��
��3��Na2SO3�ױ�������BaSO3������ˮ��������ϡHCl��
��4�������������ⷴӦ�����ӷ���ʽΪ��2S2O32-+I2=S4O62-+2I-
����[�Ʊ���Ʒ]ʵ��װ����ͼ��ʾ(ʡ�Լг�װ��)

ʵ�鲽�裺
��1������ͼ��ʾ��װ��װ�ú�Ӧ��_______________(���������)���ٰ�ͼʾ�����Լ�������B��D��������____________________________��E�е��Լ���__________(ѡ��������ĸ���)��
A��ϡH2SO4 B��NaOH��Һ C������NaHSO3��Һ
��2��������ƿC�м���Na2S��Na2CO3�����Һ��������ƿA�еμ�ŨH2SO4��C�з�Ӧ����Na2S2O3��CO2����ѧ����ʽΪ______________________��
��3����Na2S��Na2CO3��ȫ���ĺ�����Ӧ������C�л��Һ����Һ���������ᾧ�����ˡ�ϴ�ӡ�����õ���Ʒ��
����[̽���뷴˼]
��1��Ϊ��֤��Ʒ�к���Na2SO3��Na2SO4����С�����������ʵ�鷽�����뽫��������������(�����Լ���ϡHNO3��ϡH2SO4��ϡHCl������ˮ��ѡ��)��
ȡ������Ʒ���ϡ��Һ���μ�����BaCl2��Һ���а�ɫ�������ɣ�____________��������δ��ȫ�ܽ⣬���д̼�����ζ��������������ȷ����Ʒ�к���Na2SO3��Na2SO4��
��2����I2�ı���Һ�ⶨ��Ʒ�Ĵ���
ȡ10.0g��Ʒ�����Ƴ�100mL��Һ��������Һ������ˮ���뾭����С���ȴ�����ʹ�ã���Ŀ����ɱ������__________��������̼��ȡ10.00mL��Һ����________��ҺΪָʾ������Ũ��Ϊ0.10mol/LI2�ı��ܲ����еζ���������ݼ�¼���±���ʾ��
��� | 1 | 2 | 3 |
��Һ�����/mL | 10.00 | 10.00 | 10.00 |
����I2����Һ�����/mL | 19.95 | 17.10 | 20.05 |
�ζ�ʱ���ﵽ�ζ��յ��������___________________________________________��Na2S2O3��5H2O�ڲ�Ʒ�е�����������_______________(�ðٷ�����ʾ���ұ���1λС��)��







