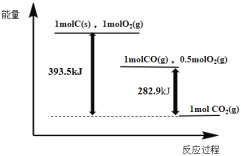
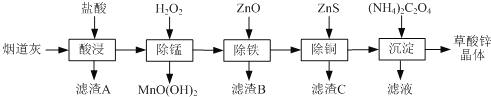
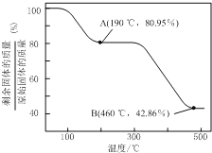
��Ŀ����
����Ŀ��������ʵ������õ���ʵ��������������ȷ����
ʵ����� | ʵ���������� | |
A | ��ij��Һ�м���ϡ���ᣬ���ɵ���ɫ�������д̼�����ζ������ | ����Һ��һ������S2O32- |
B | ��3ml KI��Һ�еμӼ�����ˮ�����ٵμ�1mL������Һ����Һ����ɫ | �����ԣ�Br2��I2 |
C | ��ͬ�����£��ⶨ��Ũ�ȵ�Na2CO3��Һ��Na2SO4��Һ��pH��ǰ�߳ʼ��ԣ����߳����� | �ǽ����ԣ�S��C |
D | ��þ����ȼ��Ѹ�����뼯��CO2�ļ���ƿ | ����ƿ�в���Ũ�̲��к�ɫ�������� |
A.AB.BC.CD.D
���𰸡�A
��������
A. ��ij��Һ�м���ϡ���ᣬ���ɵ���ɫ�������д̼�����ζ�����壬ԭ��Һ�п��ܺ���S2-��SO32-����SO32-����������ϡ����ʱ������Ӧ![]() ��
��![]() ��S�����ǵ���ɫ�����������������д̼�����ζ���壬��A����
��S�����ǵ���ɫ�����������������д̼�����ζ���壬��A����
B. ������ԭ��Ӧ�У��������������Դ�����������������ԣ���Һ����ɫ��˵���еⵥ�����ɣ�Br2������I-����I2��Br2����������I2�����������Br2�������Ա�I2��ǿ����B��ȷ��
C. Ԫ�صķǽ�����Խ����������������Ӧˮ��������Ծ�Խ��������ۺ���������εļ��Ծ�Խǿ����ͬ�����£��ⶨ��Ũ�ȵ�Na2CO3��Һ��Na2SO4��Һ��pH��ǰ�߳ʼ��ԣ����߳����ԣ�˵��̼��Ϊ���ᡢ����Ϊǿ�ᣬ�ɴ˵ó��ǽ�����S��C����C��ȷ��
D. ��þ����ȼ��Ѹ�����뼯��CO2�ļ���ƿ��þ������ȼ�գ���Ӧ����MgO��C������ƿ�в���Ũ�̣�MgO����С���������к�ɫ������������D��ȷ��
��ѡA��

 ���źþ���Ԫ����ĩ��ϵ�д�
���źþ���Ԫ����ĩ��ϵ�д� һ����ʦȨ����ҵ��ϵ�д�
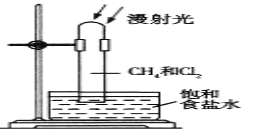
һ����ʦȨ����ҵ��ϵ�д�����Ŀ�������������Ӽ�����1��2-�������顣��ͼΪʵ�����Ʊ�1��2-���������װ��ͼ�� ͼ�з�Һ©������ƿa�зֱ�װ��ŨH2SO4����ˮ�Ҵ���dװ�D�Թ���װ��Һ�塣
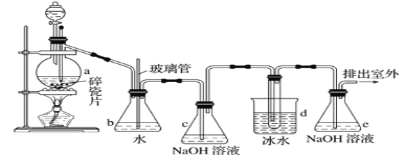
��������б����£�
�Ҵ� | 1��2-�������� | ���� | �� | |
״̬ | ��ɫҺ�� | ��ɫҺ�� | ��ɫҺ�� | ����ɫҺ�� |
�ܶ�/g��cm-3 | 0.79 | 2.18 | 0.71 | 3.10 |
�е�/�� | 78.5 | 131.4 | 34.6 | 58.8 |
�۵�/�� | -114.3 | 9.79 | - 116.2 | -7.2 |
ˮ���� | ���� | ���� | �� | ���� |
(1)��ȫƿb��ʵ�����ж������á���һ���Լ��ʵ�������dװ�D�е����Ƿ�����������д����������ʱƿb�е�������_____________________________�����ʵ��ʱdװ�D�е��ܶ���������Ϊ���ܵ�ԭ������___________________________________��
��ȫƿb����������������___________________________��
(2)����c��e�ж�ʢ��NaOH��Һ��c��NaOH��Һ��������_______________________��
(3)ijѧ��������ʵ��ʱ��ʹ��һ������Һ�壬����ȫ����ɫʱ���������Ҵ���Ũ������Һ����������ȷ����³������࣬���װ�D��������û�����⣬�Է������ܵ�ԭ��_____________________��_____________________________(д����������)��
(4)��ȥ����������δ��Ӧ��Br2�����е���Ҫ����Ϊ________________��Ҫ��һ���ᴿ�����в����б������
A���ؽᾧ B������ C����ȡ D������
(5)ʵ����Ҳ���Գ�ȥdװ����ʢ��ˮ���ձ�����Ϊ����ˮֱ�Ӽ��뵽dװ�õ��Թ��У����ʱ��ˮ����������ȴ1��2-��������������⣬��������������___________��
����Ŀ��Ϊȷ��Na2CO3��NaHCO3�������Ʒ���������ȡ�ķݸ���Ʒ����ˮ��ֱ���μ�����ͬŨ������30.0 mL����ַ�Ӧ������CO2�����(������ɱ�״���µ������������CO2��ˮ�е��ܽ�)���±���
ʵ����� | �� | �� | �� | �� |
�������(mL) | 30.0 | 30.0 | 30.0 | 30.0 |
��Ʒ����(g) | 2.96 | 3.70 | 5.18 | 6.66 |
CO2���(mL) | 672 | 840 | 896 | 672 |
(1)��Ʒ�е����ʵ���֮��n(Na2CO3)��n(NaHCO3)��________��
(2)��������ʵ���Ũ��c(HCl)��________��