��Ŀ����
Na��Mg��Al�ֱ������ᷴӦ��?
��1�������������������ʵ�����Na��Mg��Al��������������֮��Ϊ_______________��
��2����������������������Na��Mg��Al����H2������֮��Ϊ_______________����Һ��������֮��Ϊ_______________��?
��3�����������������������ַ�Ӧ��������Һ������ȣ���Na��Mg��Al����֮��Ϊ_______________��?
��4��������Ũ����ȣ������ȣ�Ͷ���Na��Mg��Al������ȣ���Ӧ����һ�ֽ���ʣ�࣬�ý�����_______________��?
��5����ȡNa��Mg��Al��m g���������·ֱ���V L 4 mol��L��1�������ַ�Ӧ�����ƶϣ���������������£�V��ȡֵ��Χ���ú�m�ı���ʽ��ʾ����
��Al�����ᷴӦ�ų�H2���ʱ��______________��?
��Na�����ᷴӦ�ų�H2���ʱ��______________��?
��Mg��Al�����ᷴӦ����H2һ����ʱ��______________��?
��1�������������������ʵ�����Na��Mg��Al��������������֮��Ϊ_______________��
��2����������������������Na��Mg��Al����H2������֮��Ϊ_______________����Һ��������֮��Ϊ_______________��?
��3�����������������������ַ�Ӧ��������Һ������ȣ���Na��Mg��Al����֮��Ϊ_______________��?
��4��������Ũ����ȣ������ȣ�Ͷ���Na��Mg��Al������ȣ���Ӧ����һ�ֽ���ʣ�࣬�ý�����_______________��?
��5����ȡNa��Mg��Al��m g���������·ֱ���V L 4 mol��L��1�������ַ�Ӧ�����ƶϣ���������������£�V��ȡֵ��Χ���ú�m�ı���ʽ��ʾ����
��Al�����ᷴӦ�ų�H2���ʱ��______________��?
��Na�����ᷴӦ�ų�H2���ʱ��______________��?
��Mg��Al�����ᷴӦ����H2һ����ʱ��______________��?
��1��1��2��3 ?
��2��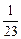 ��
��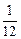 ��
��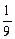
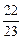 ��
��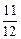 ��
��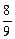
��3��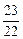 ��
��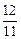 ��
��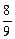
��4��Al?
��5����V��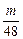 L ��V��
L ��V��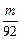 L ��V��
L ��V��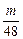 L
L
��2��
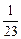 ��
��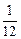 ��
��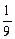
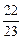 ��
��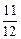 ��
��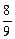
��3��
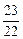 ��
��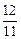 ��
��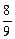
��4��Al?
��5����V��
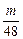 L ��V��
L ��V��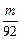 L ��V��
L ��V��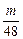 L
L��1�����������������й�ϵ��?
2Na+2HCl====2NaCl+H2��
1 mol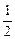 mol?
mol?
Mg+2HCl====MgCl2+H2��
1 mol 1 mol
2Al+6HCl====2AlCl3+3H2��?
1 mol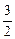 mol?
mol?
����Na��Mg��Al������������֮��Ϊ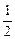 ��1��
��1��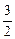 =1��2��3��
=1��2��3��
��2��2Na+2HCl====2NaCl+H2��?
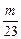 mol
mol 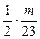 mol?
mol?
Mg+2HCl====MgCl2+H2��?
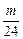 mol
mol 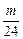 mol?
mol?
2Al+6HCl====2AlCl3+3H2��
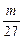 mol
mol 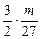 mol?
mol?
����Na��Mg��Al������������֮��Ϊ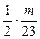 mol��
mol��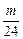 mol��
mol��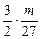 mol=
mol=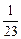 ��
��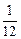 ��
��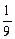 ��
��
��3��������֪��Һ����������ͬ�������Na��Mg��Al�������ֱ�Ϊx��y��z��
2Na+2HCl====2NaCl+H2��?
2 mol 1 mol?
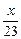 mol
mol 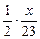 mol?
mol?
Mg+2HCl====MgCl2+H2��?
1 mol 1 mol?
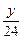 mol
mol 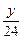 mol?
mol?
2Al+6HCl====2AlCl3+3H2��?
2 mol 3 mol?
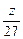 mol
mol 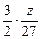 mol?
mol?
��Na�����ᷴӦ����Һ���أ�x��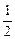 ��
��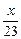 ��2=
��2=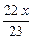 g?
g?
Mg�����ᷴӦ����Һ���أ�y��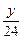 ��2=
��2=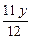 g?
g?
Al�����ᷴӦ����Һ���أ�z��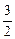 ��
��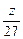 ��2=
��2=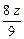 g
g
����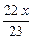 =
=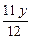 =
=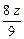
x��y��z=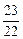 ��
��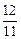 ��
��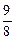
��4����2Na+2HCl====2NaCl+H2��
Mg+2HCl====MgCl2+H2��?
2Al+6HCl====2AlCl3+3H2��?
֪��������Na��Mg��Al��Al����HCl��࣬���Ե�ֻ��һ�ֽ���ʣ��ʱ��ֻ����Al��
��5����Na��Mg��Al m g����HCl�������4 mol��L��1����
2Na + 2HCl====2NaCl+H2��
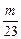 mol
mol 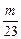 mol?
mol?
V��HCl��=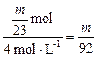 L?
L?
Mg + 2HCl====MgCl2+H2��
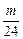 mol
mol 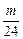 ��2 mol?
��2 mol?
V��HCl��=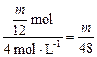 L?
L?
2Al + 6HCl====2AlCl3+3H2��?
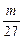
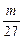 ��3 mol?
��3 mol?
V��HCl��=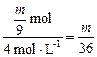 L?
L?
�ٱ�֤��m��Na��Mg��Al�����ᷴӦ��Al������H2��࣬��Na��Mg��ʣ�࣬HCl�������ɣ���V��HCl����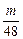 L��?
L��?
�ڱ�֤��m��Na��Mg��Al�����ᷴӦ��Na����H2��࣬��Na������HCl���㣬��V��HCl����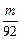 L��?
L��?
�۵�������Na��Mg��Al�����ᷴӦ��Mg��Al����H2һ���࣬��Mg��Al�����ᷴӦʱ��HCl�������Mgǡ�÷�Ӧ��ȫ����V��HCl����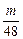 L��?
L��?
2Na+2HCl====2NaCl+H2��
1 mol
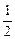 mol?
mol?Mg+2HCl====MgCl2+H2��
1 mol 1 mol
2Al+6HCl====2AlCl3+3H2��?
1 mol
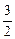 mol?
mol?����Na��Mg��Al������������֮��Ϊ
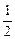 ��1��
��1��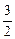 =1��2��3��
=1��2��3����2��2Na+2HCl====2NaCl+H2��?
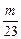 mol
mol 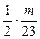 mol?
mol?Mg+2HCl====MgCl2+H2��?
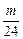 mol
mol 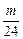 mol?
mol?2Al+6HCl====2AlCl3+3H2��
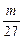 mol
mol 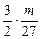 mol?
mol?����Na��Mg��Al������������֮��Ϊ
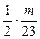 mol��
mol��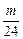 mol��
mol��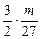 mol=
mol=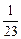 ��
��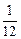 ��
��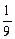 ��
����3��������֪��Һ����������ͬ�������Na��Mg��Al�������ֱ�Ϊx��y��z��
2Na+2HCl====2NaCl+H2��?
2 mol 1 mol?
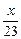 mol
mol 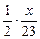 mol?
mol?Mg+2HCl====MgCl2+H2��?
1 mol 1 mol?
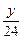 mol
mol 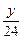 mol?
mol?2Al+6HCl====2AlCl3+3H2��?
2 mol 3 mol?
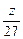 mol
mol 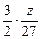 mol?
mol?��Na�����ᷴӦ����Һ���أ�x��
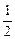 ��
��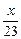 ��2=
��2=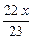 g?
g?Mg�����ᷴӦ����Һ���أ�y��
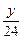 ��2=
��2=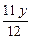 g?
g?Al�����ᷴӦ����Һ���أ�z��
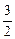 ��
��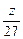 ��2=
��2=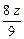 g
g����
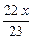 =
=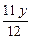 =
=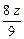
x��y��z=
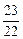 ��
��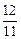 ��
��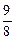
��4����2Na+2HCl====2NaCl+H2��
Mg+2HCl====MgCl2+H2��?
2Al+6HCl====2AlCl3+3H2��?
֪��������Na��Mg��Al��Al����HCl��࣬���Ե�ֻ��һ�ֽ���ʣ��ʱ��ֻ����Al��
��5����Na��Mg��Al m g����HCl�������4 mol��L��1����
2Na + 2HCl====2NaCl+H2��
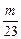 mol
mol 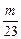 mol?
mol?V��HCl��=
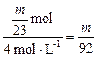 L?
L?Mg + 2HCl====MgCl2+H2��
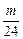 mol
mol 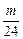 ��2 mol?
��2 mol?V��HCl��=
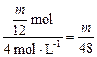 L?
L?2Al + 6HCl====2AlCl3+3H2��?
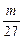
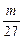 ��3 mol?
��3 mol?V��HCl��=
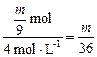 L?
L?�ٱ�֤��m��Na��Mg��Al�����ᷴӦ��Al������H2��࣬��Na��Mg��ʣ�࣬HCl�������ɣ���V��HCl����
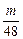 L��?
L��?�ڱ�֤��m��Na��Mg��Al�����ᷴӦ��Na����H2��࣬��Na������HCl���㣬��V��HCl����
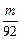 L��?
L��?�۵�������Na��Mg��Al�����ᷴӦ��Mg��Al����H2һ���࣬��Mg��Al�����ᷴӦʱ��HCl�������Mgǡ�÷�Ӧ��ȫ����V��HCl����
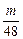 L��?
L��?
��ϰ��ϵ�д�
 ��Ӣ���㿨ϵ�д�
��Ӣ���㿨ϵ�д� Ӧ����㲦ϵ�д�
Ӧ����㲦ϵ�д�
�����Ŀ

 NA
NA

 %?
%?