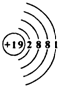
��Ŀ����
ij����С���о������ص����ʡ���������ͨ�����������Ƶ�ԭ�ӽṹʾ��ͼ�������й����ϣ�֪������������ͬһ�����ʣ�������������������֮�������ڽ������˳����У��������Ƶ�ǰ�棬���ڿ�����ȼ�յIJ����dz������أ�KO2����
����������⣺
��1���ص�ԭ�Ӻ�����19�����ӣ������ص�ԭ�ӽṹʾ��ͼ_______________��
��2��Ԥ��صĻ�ѧ���ʣ�������з�Ӧ�Ļ�ѧ����ʽ��
�ٵ�ȼ�ؾ���ȼ��_____________________________________________________
�ڽ�С���Ͷ����ˮ��________________________________________________
��3���Ʋ������Ȼ���еĴ��ڷ�ʽ��____________�������̬������̬����
��1��
��2����K+O2 ![]() KO2 ��2K+2H2O = 2KOH+H2��
KO2 ��2K+2H2O = 2KOH+H2��
��3������̬

��ϰ��ϵ�д�
 �Ͻ�ƽ���Ȿϵ�д�
�Ͻ�ƽ���Ȿϵ�д� ����ѧ��Ӧ�����ϵ�д�
����ѧ��Ӧ�����ϵ�д�
�����Ŀ