ЬтФПФкШн
ЁОЬтФПЁПбЮФрЪЧТШМюЙЄвЕжаЕФЗЯдќЃЌжївЊГЩЗжЪЧУОЕФЙшЫсбЮКЭЬМЫсбЮ(КЌЩйСПЬњЁЂТСЁЂИЦЕФбЮ)ЁЃЪЕбщЪввдбЮФрЮЊдСЯжЦШЁMgSO4ЁЄ7H2OЕФЪЕбщЙ§ГЬШчЯТЃК
![]()
![]()
![]()
![]() ЈDЁњ
ЈDЁњ![]() ЈDЁњВњЦЗ
ЈDЁњВњЦЗ
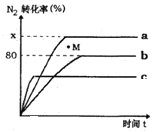
вбжЊЃКЂйЪвЮТЯТKsp[Mg(OH)2]ЃН6.0ЁС10Ѓ12ЁЃЂкдкШмвКжаЃЌFe2ЃЋЁЂFe3ЃЋЁЂAl3ЃЋДгПЊЪМГСЕэЕНГСЕэЭъШЋЕФpHЗЖЮЇвРДЮЮЊ7.1ЁЋ9.6ЁЂ2.0ЁЋ3.6ЁЂ3.1ЁЋ4.7ЁЃЂлШ§жжЛЏКЯЮяЕФШмНтЖШ(S)ЫцЮТЖШБфЛЏЕФЧњЯпШчЭМЫљЪОЁЃ

(1)гЩТЫвКЂёЕНТЫвКЂђашЯШМгШыNaClOЕїШмвКpHдМЮЊ5ЃЌдйГУШШЙ§ТЫЃЌдђГУШШЙ§ТЫЕФФПЕФЪЧ________ЃЌТЫдќЕФжївЊГЩЗжЪЧ________ЁЃ
(2)ДгТЫвКЂђжаЛёЕУMgSO4ЁЄ7H2OОЇЬхЕФЪЕбщВНжшвРДЮЮЊЂйЯђТЫвКЂђжаМгШы___________ЃЛЂкЙ§ТЫЃЌЕУГСЕэЃЛЂл____________________ЃЛЂмеєЗЂХЈЫѕЃЌНЕЮТНсОЇЃЛЂнЙ§ТЫЁЂЯДЕгЕУВњЦЗЁЃ
(3)ШєЛёЕУЕФMgSO4ЁЄ7H2OЕФжЪСПЮЊ24.6 gЃЌдђИУбЮФржаУО[вдMg(OH)2МЦ]ЕФАйЗжКЌСПдМЮЊ____________(MgSO4ЁЄ7H2OЕФЯрЖдЗжзгжЪСПЮЊ246)ЁЃ
ЁОД№АИЁП(1)ЮТЖШНЯИпЪБИЦбЮгыУОбЮЗжРыЕУИќГЙЕз(ЛђИпЮТЯТCaSO4ЁЄ2H2OШмНтЖШаЁЕШКЯРэД№АИОљПЩ) Al(OH)3ЁЂFe(OH)3ЁЂCaSO4ЁЄ 2H2O (2)NaOHШмвК ЯђГСЕэжаМгзуСПЯЁСђЫс(ЦфЫћКЯРэД№АИОљПЩ)
(3)20.0%
ЁОНтЮіЁП(1)МгШыNaClOЃЌПЩвдАбFe2ЃЋбѕЛЏГЩFe3ЃЋЃЌЕБpHДѓдМЮЊ5ЪБЃЌFe3ЃЋЁЂAl3ЃЋзЊЛЏГЩГСЕэЃЛИљОнШмНтЖШЧњЯпЃЌЮТЖШИпЪБЃЌCaSO4ЁЄ2H2OЕФШмНтЖШИќаЁЃЌЫљвдТЫдќЕФжївЊГЩЗжЮЊAl(OH)3ЁЂFe(OH)3КЭCaSO4ЁЄ2H2OЁЃ(2)ЪЙMg2ЃЋзЊЛЏГЩMgSO4ЁЄ7H2OЕФВНжшЮЊЂйМгШыNaOHШмвКЃЛЂкЙ§ТЫЕУMg(OH)2ГСЕэЃЛЂлМгШыЯЁH2SO4ЃЛЂмеєЗЂХЈЫѕЁЂНЕЮТНсОЇЁЂЙ§ТЫЕУВњЦЗЁЃ(3)ИљОнУОдЊЫиЪиКуЕУ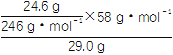 ЁС100%ЃН20%ЁЃ
ЁС100%ЃН20%ЁЃ

ЁОЬтФПЁПФГКЯН№гыЬњЕФЮяРэаджЪЕФБШНЯШчЯТБэЫљЪОЃК
ЁЁаджЪ жжРрЁЁ ЁЁ | ШлЕу | УмЖШ | гВЖШ | ЕМЕчад |
ФГКЯН№ | 3 200Ёц | 3.20 g/cm3 | 7.5 | 3.5 |
Ьњ | 1 535Ёц | 7.86 g/cm3 | 4.5 | 17 |
(зЂЃКгВЖШвдН№ИеЪЏЮЊ10зїБъзМЃЌЕМЕчадвдвјЕФЕМЕчад100ЮЊБъзМ)
вбжЊИУКЯН№ФЭИЏЪДЁЂЧПЖШДѓЃЌДгадФмПДЃЌИУКЯН№ВЛЪЪКЯжЦ
A. УХДАПђB. ТЏОп
C. ЕМЯпD. ЗЩЛњЭтПЧ