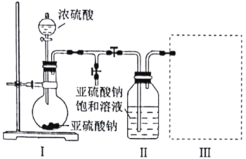
��Ŀ����
����Ŀ�������ɶ�����Ⱦ���γɣ����������PM2.5)����������(NOx)��CO��SO2�ȡ���ѧ�ڽ��������Ⱦ������������Ҫ�����á�
(1)��֪����2CO(g)+O2(g)=2CO2(g)��H1=��566.0kJ��mol��1
��N2(g)+2O2(g)=2NO2(g)��H2=+64kJ��mol��1
��Ӧ2NO2(g)+4CO(g=N2(g)+4CO2(g)��__________(����¡����¡�)���������Է����С�
(2)�о���������NH3�ɳ�ȥ���Ṥҵβ���е�NO��NH3��NO�����ʵ���֮�ȷֱ�Ϊ1��2��1��1.5��3��1ʱ��NO�ѳ������¶ȱ仯��������ͼ1��ʾ��

������a�У�NO����ʼŨ��Ϊ6��10��4mg��m��3������X�㵽Y�㾭��20s�����ʱ�����NO���ѳ�����Ϊ_________mg��m��3��s��1��
������c��Ӧ��NH3��NO�����ʵ���֮����________����������__________
(3)̿���������е���Ҫ������о����������Ի�����ӣ����ɻ����������Կ�������SO2��������е������仯ģ���������ͼ2��ʾ����ˮ����£�һ�������ӵĻ��Ϊ__________����������ӵ�������_________(���ˮ������ˮ��)��
(4)����Ӧ2NO(g)+2CO(g)![]() N2(g)+2CO2(g)�������淴Ӧ���ʷֱ�ɱ�ʾΪv��=k��c2(NO)��c2(CO)��v��=k��c(N2)��c2(CO2)��k����k���ֱ�Ϊ�����淴Ӧ���ʳ�����cΪ���ʵ���Ũ�ȡ�һ���¶��£������Ϊ1L�ĺ����ܱ������м���4molNO��4molCO����������Ӧ�����CO��CO2�����ʵ���Ũ����ʱ��ı仯��ͼ3��ʾ��
N2(g)+2CO2(g)�������淴Ӧ���ʷֱ�ɱ�ʾΪv��=k��c2(NO)��c2(CO)��v��=k��c(N2)��c2(CO2)��k����k���ֱ�Ϊ�����淴Ӧ���ʳ�����cΪ���ʵ���Ũ�ȡ�һ���¶��£������Ϊ1L�ĺ����ܱ������м���4molNO��4molCO����������Ӧ�����CO��CO2�����ʵ���Ũ����ʱ��ı仯��ͼ3��ʾ��

��a��ʱ��v����v��=_____________��
�ڲ��ƽ��ʱ��ϵѹǿΪp��KPΪ�������ѹ��ʾ��ƽ�ⳣ������ѹ=��ѹ�����ʵ�����������ƽ�ⳣ��KP=____________(�ú�p��ʽ�ӱ�ʾ)��
(5)ij�о�С��̽���÷�Ӧ�д������ѵ���(NO��ת����)��Ӱ�졣�������ʵ�����NO��CO��һ�������ٷֱ�ͨ������a��b��������Ӧ2NO(g)+2CO(g)![]() N2(g)+2CO2(g)����ͬʱ�䡢��ͬ�¶��²��ʹ�ô���aʱ�ѵ������¶ȵĹ�ϵ��ͼ4�����ߢ���ʾ����֪��Ч��b��a������b�Ļ�����450��ʱ���(δ��ƽ��)������ͼ4�л���ʹ�ô���bʱ����Ӧ������(��300�濪ʼ��)��_______________
N2(g)+2CO2(g)����ͬʱ�䡢��ͬ�¶��²��ʹ�ô���aʱ�ѵ������¶ȵĹ�ϵ��ͼ4�����ߢ���ʾ����֪��Ч��b��a������b�Ļ�����450��ʱ���(δ��ƽ��)������ͼ4�л���ʹ�ô���bʱ����Ӧ������(��300�濪ʼ��)��_______________
���𰸡����� 6��10��6 1��2 �¶���ͬʱ��NH3��NO���ʵ����ı�ֵԽС��NO���ѳ���ԽС 0.75eV ��ˮ 1��160(![]() ��0.00625)
��0.00625) ![]()
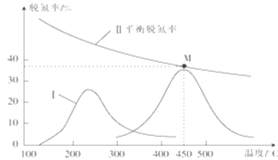 (450��ʱ������ߵ㲻����M��)
(450��ʱ������ߵ㲻����M��)
��������
���ݸ�˹���ɣ��������Ӧ���ʱ䣬�����Է���Ӧ����:��H-T��S��0�жϸ÷�ӦҪ�ڵ��������²����Է����У�
�ѳ�����Ϊ:�仯������Ũ��/�仯��ʱ�䣻�¶���ͬʱ��NH3��NO�����ʵ����ı�ֵԽС��NO���ѳ���ԽС��
������ӵĻ��Խ�ͣ�Խ��������ӣ�
����ƽ��ʱ������ʽ��ƽ��ʱv��=v�������![]() ���ٸ���a��ʱ������ʽ�����ݿɼ����v��:v��= k��c(N2)��c2(CO2):k��c2(NO)��c2(CO)=
���ٸ���a��ʱ������ʽ�����ݿɼ����v��:v��= k��c(N2)��c2(CO2):k��c2(NO)��c2(CO)=![]() ��
��
����b�Ļ�����450��ʱ���(δ��ƽ��)�����ѵ�����ɻ�������Ӧ�����ߡ�
(1)�ɷ�Ӧ�ٺ͢ڣ����ݸ�˹���ɣ��������Ӧ2NO2(g)+4CO(g)=N2(g)+4CO2(g)���ʱ�Ϊ-1196 kJ��mol��1��0����һ���ؼ��ķ�Ӧ��������S��0�������Է���Ӧ����:��H-T��S��0�����Ը÷�ӦҪ�ڵ��������²����Է����У�
(2)������a�У�NO����ʼŨ��Ϊ6��10��4mg��m��3������X�㵽Y�㾭��20s�����ʱ�����NO���ѳ�����Ϊ��![]() mg��m��3��s��1��
mg��m��3��s��1��
���¶���ͬʱ��NH3��NO�����ʵ����ı�ֵԽС��NO���ѳ���ԽС������a��b��c���߶�Ӧ��NH3��NO�����ʵ���֮�ȷֱ�Ϊ:3:1��1:1.5��1:2����������c��Ӧ��NH3��NO�����ʵ���֮����1:2��
(3)��ͼ��֪����ˮ����£�һ�������ӵĻ��ΪΪ0.75eV����ͼ�е����ݿ��Կ���������ˮ�����£�������ӵĻ�ܽϵͣ�������������ӵ���������ˮ������
(4)��a��ʱ���г�����ʽΪ:
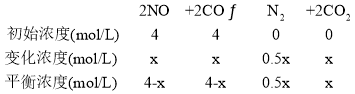
��ΪCO��CO2�����ʵ���Ũ����ȣ���4-x=x�����x=2��
ƽ��ʱ�У�
����ƽ��ʱ������ʽ��ƽ��ʱv��=v�������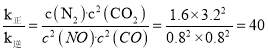 ����a��ʱv��:v��= k��c(N2)��c2(CO2):k��c2(NO)��c2(CO)=
����a��ʱv��:v��= k��c(N2)��c2(CO2):k��c2(NO)��c2(CO)=![]() ��
��
��ƽ��ʱ��ϵѹǿΪp��KPΪ�������ѹ��ʾ��ƽ�ⳣ�������ʵ���Ũ��Ϊ0.8+0.8+1.6+3.2=6.4 mol/L�����ݷ�ѹ=��ѹ�����ʵ�����������ƽ�ⳣ��KP= ��
��
(5)����b�Ļ�����450��ʱ���(δ��ƽ��)����ͼ4��ʹ�ô���bʱ����Ӧ��������ͼ: ��
��

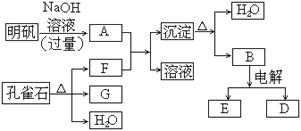
����Ŀ���ο�����ͼ�����й�Ҫ��ش����⣺
��1��ͼ����1molNO2(g)��1molCO(g)��Ӧ����CO2��NO�����������仯ʾ��ͼ�����ڷ�Ӧ��ϵ�м����������Ӧ��������E2�ı仯��__(�������С�����䡱����ͬ)����H�ı仯��__����д��NO2��CO��Ӧ���Ȼ�ѧ����ʽ��__��
��2���±��Dz��ֻ�ѧ���ļ������ݣ�
��ѧ�� | P��P | P��O | O=O | P=O |
����(kJ��mol-1) | 198 | 360 | 498 | x |
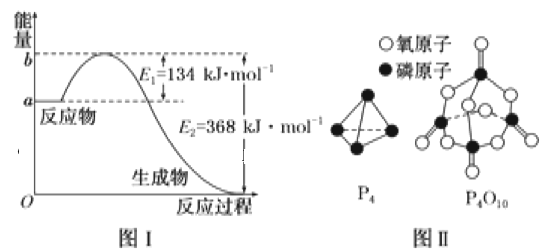
��֪1mol����(P4)��ȫȼ�շ���Ϊ1194kJ����������ȫȼ�յIJ���ṹ��ͼ����ʾ�������x=__kJ��mol-1��
��3��PCl5��һ����Ҫ�ĺ���������л��ϳ��������Ȼ�����ij�¶�ʱ����2.0L���º����ܱ������г���1.0molPCl5��������ӦPCl5(g)![]() PCl3(g)+C12(g)��H=+124kJ��mol-1����Ӧ�����вⶨ�IJ������ݼ��±���
PCl3(g)+C12(g)��H=+124kJ��mol-1����Ӧ�����вⶨ�IJ������ݼ��±���
ʱ��t/s | 0 | 50 | 150 | 250 | 350 |
n(PCl3��/mol | 0 | 0.16 | 0.19 | 0.2 | 0.2 |
��Ӧ��ǰ50s��ƽ������v(PCl5)=__���ڸ��¶��£�����ʼʱ����0.5molPCl5��amolCl2��ƽ��ʱPCl5��ת������Ϊ20������a=__��