��Ŀ����
(9��)��һ�λ�ѧ��ȤС���У�һС���������ʼ以�䣬��Ƴ�һƽ�桰ħ��������ͼ��ʾ��

��֪����A��B��C��D��G����ͬһ��Ԫ�ء�
���������
��E��ͨ��������ܶ���С�����壻B����������Һ��Ӧ���ɲ�����ϡ����İ�ɫ��������ˮ��Һ��BҲ�ܽ�һ����������������ΪF��F�Ǻ�������Ԫ�صĻ������A��Ӧ����E��G��
����������Ϣ��գ�
(1)д��D�Ļ�ѧʽ________��G����C�Ĺ����������ֵ�����Ϊ________________________________________________________________________��
(2)д�����з�Ӧ�Ļ�ѧ����ʽ��E��A________��A��B________��
(3)B��F�����ӷ���ʽΪ_________________________________________________��

��֪����A��B��C��D��G����ͬһ��Ԫ�ء�
���������
| ���� | ��A(����) | B(��Һ) | D(����) | G(��Һ) |
| ��ɫ | ����ɫ | ��ɫ | ����ɫ | dz��ɫ |
����������Ϣ��գ�
(1)д��D�Ļ�ѧʽ________��G����C�Ĺ����������ֵ�����Ϊ________________________________________________________________________��
(2)д�����з�Ӧ�Ļ�ѧ����ʽ��E��A________��A��B________��
(3)B��F�����ӷ���ʽΪ_________________________________________________��
(1)Fe2O3���Ȳ�����ɫ��������Ϊ����ɫ������Ϊ���ɫ
(2)4H2��Fe3O4��3Fe��4H2O
2Fe��3Cl2��ȼ2FeCl3
(3)2Fe3����SO2��2H2O===2Fe2����SO42����4H��
(2)4H2��Fe3O4��3Fe��4H2O
2Fe��3Cl2��ȼ2FeCl3
(3)2Fe3����SO2��2H2O===2Fe2����SO42����4H��
�𰸣�(1)Fe2O3���Ȳ�����ɫ��������Ϊ����ɫ������Ϊ���ɫ
(2)4H2��Fe3O4��3Fe��4H2O
2Fe��3Cl2��ȼ2FeCl3
(3)2Fe3����SO2��2H2O===2Fe2����SO42����4H��
�⣺�����⣬
| ���� | ��A(����) | B(��Һ) | D(����) | G(��Һ) |
| ��ɫ | ����ɫ | ��ɫ | ����ɫ | dz��ɫ |
| | Fe | FeCl3 | Fe2O3 | FeSO4 |
A�DFe,B�DFeCl3,C�DFe (OH)3 , D�DFe2O3 ,E�DH2 ,F�DH2SO4 ,G�DFe SO4
(1)�Ȳ�����ɫ��������Ϊ����ɫ������Ϊ���ɫ
BΪ����ɫFe2O3��G����C��Fe2����2OH�D="Fe" (OH)2 ,4Fe(OH)2��O2��2H2O="4Fe" (OH)3,,�������Ȳ�����ɫ��������Ϊ����ɫ������Ϊ���ɫ
(2) E��A �ķ���ʽ��4H2��Fe3O4��3Fe��4H2O
A��B�ķ���ʽ��2Fe��3Cl2��ȼ2FeCl3
(3) B��F�����ӷ���ʽΪ��2Fe3����SO2��2H2O===2Fe2����SO42����4H��

��ϰ��ϵ�д�
 �����Ļ�������ҵϵ�д�
�����Ļ�������ҵϵ�д�
�����Ŀ
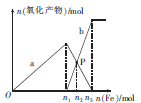
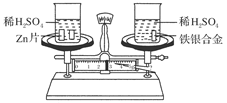
 eCO3��ijѧϰС����������ʵ�飺
eCO3��ijѧϰС����������ʵ�飺 ��������ʲô��
��������ʲô�� �����������ʹ�õĽ���֮һ�����������仯�����֪ʶ������������⡣
�����������ʹ�õĽ���֮һ�����������仯�����֪ʶ������������⡣
 Al(OH)4- + H+ ��NH3+H2O
Al(OH)4- + H+ ��NH3+H2O