��Ŀ����
��������Ĺ���ʮ�ָ��ӣ���֪ij���⺬��Fe2O3?nH2O(n��1)����������Fe(OH)3��F eCO3��ijѧϰС����������ʵ�飺
eCO3��ijѧϰС����������ʵ�飺
��ȡ12.574g��������������������أ��õ�����10.528g��
����ȡ6.287g���⣬��205.00 mL 1.000mol/L��ϡ������ǡ����ȫ�ܽ⣬����NO����89.60 mL(��״��)��
��1������ʵ��٣��������Ƿ�ֻ��Fe2O3?nH2O����ͨ������ش�
��2������ʵ��ڣ��ܷ�ȷ�������к���FeCO3 ��������ʲô��
��������ʲô��
��3����n=0.8����ͨ������ȷ��6.287g����ijɷ��Լ����ǵ����ʵ�����
 eCO3��ijѧϰС����������ʵ�飺
eCO3��ijѧϰС����������ʵ�飺��ȡ12.574g��������������������أ��õ�����10.528g��
����ȡ6.287g���⣬��205.00 mL 1.000mol/L��ϡ������ǡ����ȫ�ܽ⣬����NO����89.60 mL(��״��)��
��1������ʵ��٣��������Ƿ�ֻ��Fe2O3?nH2O����ͨ������ش�
��2������ʵ��ڣ��ܷ�ȷ�������к���FeCO3
 ��������ʲô��
��������ʲô����3����n=0.8����ͨ������ȷ��6.287g����ijɷ��Լ����ǵ����ʵ�����
����6�֣�
��1����;��ֻ��Fe2O3?nH2O����Fe2O3��H2O�����ʵ���֮��Ϊ
10.528��160/��12.574-10.528����18=0.0658��0.114=1��1.72 n��1���������⣨2�֣��������ɣ� ��2���У���Ϊֻ��FeCO3�Ի�ԭ�ԣ�1�֣��������ɣ�
��3��Fe2O3?0.8H2O��0.025 mol��Fe(OH)3��0.005 mol��FeCO3��0.012mol����1�֣�
��1����;��ֻ��Fe2O3?nH2O����Fe2O3��H2O�����ʵ���֮��Ϊ
10.528��160/��12.574-10.528����18=0.0658��0.114=1��1.72 n��1���������⣨2�֣��������ɣ� ��2���У���Ϊֻ��FeCO3�Ի�ԭ�ԣ�1�֣��������ɣ�
��3��Fe2O3?0.8H2O��0.025 mol��Fe(OH)3��0.005 mol��FeCO3��0.012mol����1�֣�
��

��ϰ��ϵ�д�
 һ����ʦȨ����ҵ��ϵ�д�
һ����ʦȨ����ҵ��ϵ�д�
�����Ŀ

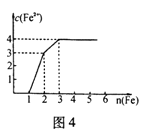

 ��ʹ�õ��IJ����������ձ���©���⣬����________________________
��ʹ�õ��IJ����������ձ���©���⣬����________________________ ��
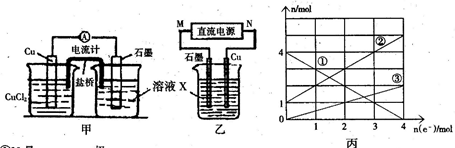
��
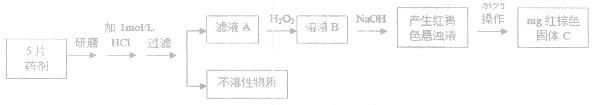
 ʱ�����ڿ����У����桰���ơ������û�ѧ����ʽ��ʾԭ�� ��
ʱ�����ڿ����У����桰���ơ������û�ѧ����ʽ��ʾԭ�� ��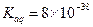 �Լ�������ʳ�����ȫ����Ҫ��pH= ��Ҫ����2λ��Ч���֣�����֪��Һ������Ũ��С��10-5mol/Lʱ�������ӿɿ���������ȫ��lg2=0.3��
�Լ�������ʳ�����ȫ����Ҫ��pH= ��Ҫ����2λ��Ч���֣�����֪��Һ������Ũ��С��10-5mol/Lʱ�������ӿɿ���������ȫ��lg2=0.3��