��Ŀ����
����Ŀ��ʵ���ҿ���ͭ��Ũ������Ȼ�������������Ʒ�Ӧ��ȡ��������

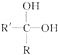
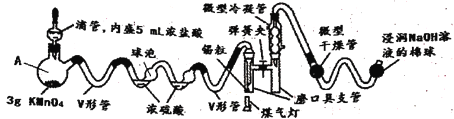
(1)�����������������Ʒ�Ӧ��ȡ��������ϣ���ܿ��Ʒ�Ӧ�ٶȣ�ͼ�п�ѡ�õķ���װ���� ______ (��д��ĸ)��
(2)����������������Ʒ�Ӧ��ȡ3.36 L(��״��)���������������40%��������(��������)���������������ƣ����������ȡ���������� ______g(����һλС��)��
(3)ij�ȵ糧�Ͽմ��������������������س��꣬�ֶԸ�������ˮ��Ʒ����̽����������pH��ֽ�ⶨ��ˮ��Ʒ��pH����������Ϊ______�������ƷpHԼΪ3��Ϊ��һ��̽����SO2���γ���������ʣ���һ������SO2ͨ������ˮ�У����pHΪ3����Һ��Ȼ����Һ��ΪA��B���ݣ�����ҺB�����ڿ����У����ܱձ����A��ȣ����ú����ҺB��ˮ�ĵ���̶Ƚ� ______(����������������С������������)��
���𰸡�ae 31.5 ȡһ����ֽ���ڸ���ྻ�ı�����(����Ƭ)�ϣ��ø���ྻ�IJ�����պȡ��ˮ��Ʒ������ֽ���룬����Ӻ����ɫ��������ձ���ɫ�������� ��С
��������
(1)�����������������ȡSO2���Լ�Ϊ��̬��Һ̬����Ӧ����������ȣ���ͨ������������������������Ʒ�Ӧ���ʣ�
(2)�����غ�ɵã�Na2SO3��SO2�����ݹ�ϵʽ��������������ʵ����������Ҫ�������Ƶ�����������������Ƶ������������ټ������Ҫ���ʺ���������Ƶ�������
(3)�ⶨpH�����ò�����պȡ��Һ��Ȼ�����ɫ���Աȣ�����ҺB�����ڿ����У������ᱻ�����������ᣬ��Һ������ǿ��
(1)��������������Ʒ�Ӧ��ȡ��������ϣ���ܿ��Ʒ�Ӧ�ٶȣ����ڷ�Ӧ����Ҫ���ȣ��ų�װ��d����������������ϸС����������ѡ��װ��c��װ��b����֪��Ӧ���ʣ��ʿ�ѡ�õķ���װ��Ϊ��ae��
(2)����������������Ʒ�Ӧ��ȡ���������ݷ�Ӧ����ʽ��Na2SO3+H2SO4=Na2SO4+SO2��+H2O�����ݷ�Ӧ����ʽ��֪��Na2SO3��SO2��n(SO2)=![]() =0.15 mol�������������Ƶ�����Ϊ��m(Na2SO3)= 0.15 mol��126 g/mol=18.9 g���������40%��������(��������)���������������ƣ����������Ƶ���������Ϊ60%���������ȡ���������Ƶ�����Ϊ
=0.15 mol�������������Ƶ�����Ϊ��m(Na2SO3)= 0.15 mol��126 g/mol=18.9 g���������40%��������(��������)���������������ƣ����������Ƶ���������Ϊ60%���������ȡ���������Ƶ�����Ϊ![]() ==31.5 g��
==31.5 g��
(3)�ⶨpH�����ò�����պȡ��Һ������pH��ֽ�ϣ�����Ӻ����ɫ���Աȣ���������Ϊȡһ����ֽ���ڸ���ྻ�ı�����(����Ƭ)�ϣ��ø���ྻ�IJ�����պȡ��ˮ��Ʒ����pH��ֽ�ϣ�����Ӻ����ɫ���ٶ��ձ���ɫ������������ҺB�����ڿ����У������ᱻ�����������ᣬ������Һ������ǿ����Һ��c(H+)����ˮ���������������ǿ����ˮ�ĵ���̶ȼ�С��

 ������ÿ�ʱ��ҵϵ�д�
������ÿ�ʱ��ҵϵ�д� ӥ�ɽ̸��νӽ̲ĺӱ�����������ϵ�д�
ӥ�ɽ̸��νӽ̲ĺӱ�����������ϵ�д�