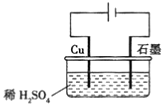
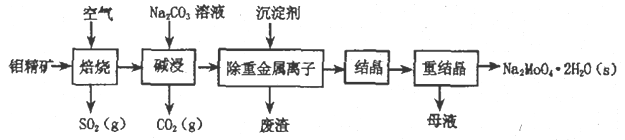
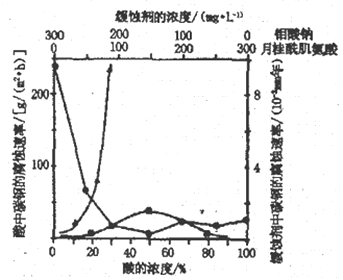
��Ŀ����
����Ŀ����ͼ��Ԫ�����ڱ���һ���֣����еĢ�~����Ԫ�أ���Ԫ�ط��Ż�ѧʽ��ջش�
| ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
�� | �� | �� | ||||||
�� | �� | �� | �� | �� | �� | �� | ||
�� | �� |
��1������ЩԪ���У���ѧ��������õ�ԭ�ӵ�ԭ�ӽṹʾ��ͼΪ��
��2���ؿ��к������Ľ���Ԫ������
��3��������γɵĻ�����ĵ���ʽ��
��4����ЩԪ���е�����������Ӧ��ˮ�����У�������ǿ���� �� ������ǿ���� �� �����Ե�������������
���𰸡�
��1��![]()
��2��Al
��3��![]()
��4��HClO4,KOH,Al(OH)3
����������Ԫ�������ڱ��е�λ�ÿ�֪����ΪN����ΪF����ΪNa����ΪMg����ΪAl����ΪSi����ΪCl����ΪAr����ΪK��
(1)����Ԫ����ֻ��Ar������������Ϊ8�����ʲ����ã����ȶ�����ԭ�ӽṹʾ��ͼΪ ![]() �����Դ��ǣ�
�����Դ��ǣ� ![]() ��
��
(2)�ؿ���Ԫ�صĺ����϶����O��Si��Al��Ca����ؿ��к������Ľ���Ԫ����Al�����Դ��ǣ�Al��
(3)������γɵĻ�����Ϊ����þ��Ϊ���ӻ���������ʽΪ ![]() �����Դ��ǣ�
�����Դ��ǣ� ![]() ��
��
(4)����Ԫ��������������Ӧ��ˮ������������ǿ��ΪHClO4��������ǿ��ΪKOH�������Ե���������ΪAl(OH)3�����Դ��ǣ�HClO4��KOH��Al(OH)3.

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�