��Ŀ����
�����£����и���������ָ����Һ�п��ܴ����������:
| A����������Ժ�ɫ����Һ�У� Na����NO3���� Fe2+��SO32�� |
| B��ˮ������� c ( H+) = 10��12 mol/L ����Һ��:K+�� AlO2���� CH3COO���� Cl�� |
C��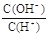 =10��12����Һ�У� K+��ClO����S2���� Cl�� =10��12����Һ�У� K+��ClO����S2���� Cl�� |
| D��c ( Fe 3+ ) =" 0" .1 mol/L ����Һ�У� K+��Cl�� ��SO42���� SCN�� |
B
�������������A����������Ժ�ɫ����ҺΪ���ԣ� NO3����Fe2+��SO32�����������棻B��ˮ�������c(H+)=10��12 mol/L����Һ����Ϊ���ԣ�AlO2����CH3COO�����ܴ������棬����Ϊ���ԣ����Դ������棻C��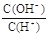 =10��12��Һ��c(H+)=10-1mol/L����������Һ��ClO����S2������������ԭ��Ӧ��D��c(Fe3+)="0.1" mol/L ����Һ�в�����SCN����Fe3+���䷢����Ϸ�Ӧ��
=10��12��Һ��c(H+)=10-1mol/L����������Һ��ClO����S2������������ԭ��Ӧ��D��c(Fe3+)="0.1" mol/L ����Һ�в�����SCN����Fe3+���䷢����Ϸ�Ӧ��
���㣺���������������鹲������жϡ�

 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д������£����и��������ܴ����������
| A��ϡ�����У�K����Mg2+��AlO2����S2O32�� |
| B��Na2S��Һ�У�SO42-��K����Cl����Cu2�� |
C��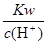 =10-13mol��L-1��Һ�У�Fe3+��NH4����Mg2+�� SO42- =10-13mol��L-1��Һ�У�Fe3+��NH4����Mg2+�� SO42- |
| D��ͨ�����CO2����Һ�У�Na+��ClO����CH3COO����HCO3�� |
�������ӷ���ʽ��д��ȷ����
| A��Ba(OH)2��Һ��ϡ���ᷴӦ��Ba2++OH-+H++SO42-=BaSO4��+H2O |
| B��Fe��FeCl3��Һ��Ӧ��Fe+Fe3+=2Fe2+ |
| C������ʯ�м���ϡ���CaCO3+2H+=Ca2++CO2��+H2O |
| D��AlCl3��Һ�мӹ�����ˮ��Al3++3OH-=Al(OH)3�� |
��H2SO4��K2SO4��Al2 (SO4)3������[KAl(SO4)2��12H2O]�Ļ����Һ�У�H����Ũ��Ϊ0.2mol/L��SO42-��Ũ��Ϊ0.5mol/L������������0.6mol/L KOH��Һʱ�����ɵij���ǡ����ȫ�ܽ⡣��Ӧ����Һ��K+��Ũ��ԼΪ
| A��0.225mol/L | B��0.25mol/L | C��0.45mol/L | D��0.55mol/L |
ij��Һ�д��ڴ�����H+��Cl����Fe3+������Һ�л����ܴ������ڵ�������
| A��OH�� | B��Ag+ | C��CO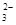 | D��SO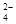 |
����ɫ��Һ�У����������ܴ����������
| A��Na+��NH4+��NO3-��MnO4- | B��Mg2+��SO42-��K +��Cl- |
| C��K +��Cu2+ ��Cl-��Br- | D��Ba2+ ��Na+ ��OH-��HCO3- |
���з�Ӧ�����ӷ���ʽ��ȷ����
| A����������Һ�м�������ϡ��� S2��+ 2H+ = H2S�� |
| B�����Ȼ�����Һ��ͨ���������壺 2Fe3++ S2��= 2Fe2++ S�� |
| C���ö��Ե缫��ⱥ��ʳ��ˮ��2Cl��+2H2Oͨ��2OH��+ H2�� + Cl2�� |
D����ȩ��������Ӧ��CH3CHO+2Ag(NH3)2OH CH3COO��+NH4++2Ag��+3NH3+H2O CH3COO��+NH4++2Ag��+3NH3+H2O |
��֪2MOyx��+5S2��+16H+=2M2+ +5S��+ 8H2O��������ѡ����ȷ����
| A��x=2 | B����������ͻ�ԭ����ı�Ϊ2:5 |
| C������4mol H2Oת��10mol���� | D��MOyx���е�M�Ļ��ϼ�Ϊ+7 |