��Ŀ����
 ��1�������£�������һԪ��ֱ��NaOH��Һ�������ϣ�ʵ���������£�
��1�������£�������һԪ��ֱ��NaOH��Һ�������ϣ�ʵ���������£�| ��� | һԪ�� | NaOH | �����Һ��pH |
| �� | c��HX��=0.1mol/L | c��NaOH��=0.1mol/L | pH=a |
| �� | c��HY��=c1 mol/L | c��NaOH��=0.1mol/L | pH=7 |
| �� | c��HZ��=0.1mol/L | c��NaOH��=0.1mol/L | pH=9 |
| �� | pH=2 HZ | pH=12 NaOH | pH=b |
������ʵ����HYΪǿ�ᣬ��HY��Һ��pH=
�۱���ʵ�鷢����Ӧ�����ӷ���ʽΪ
�ܶ���ʵ����b
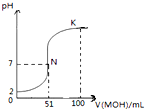
��2����100mL 0.01mol/L HA��Һ����μ���0.02mol/L MOH��Һ��ͼ����ʾ���߱�ʾ�����Һ��pH�仯���������仯���Բ��ƣ�����
����ͼ����Ϣ��֪HAΪ
��K���Ӧ����Һ�У�c��M+��+c��MOH��
���㣺�����ʱ�Ķ����жϼ��й�ph�ļ���
ר�⣺����ƽ������Һ��pHר��
��������1���ٵ��������Ũ�ȵ�HA��NaOH��Һǡ�÷�Ӧ����NaX��HAΪ���ᣬ��NaXΪǿ�������Σ���Һ��ʾ���ԣ������ε�ˮ�⼰��Һ��pH�ж���Һ������Ũ�ȴ�С��
�������е������HY���������Ʒ�Ӧ����NaY��HYΪǿ�ᣬ��Һ��pH=7������Һ��c��OH-��=c��H+����c��Na+��=c��Y-��������HY��Ũ��Ϊ0.1mol/L��
��pH=9��˵��NaZΪǿ�������Σ�HZΪ���ᣬ�������ӻ���������õ�ˮ���������������Ũ�ȣ�
��HZΪ���ᣬ��Һ��ֻ�ܲ��ֵ���������ӣ�����Һ��Ϻ����������Һ��ʾ���ԣ�
��2���ٸ������Ũ�Ⱥ���Һ��pH�ж����ǿ����
��K��ʱ����Һ��Ӧ�������Ϊ�����ʵ�����MOH��MA�����������غ�����жϣ�
�������е������HY���������Ʒ�Ӧ����NaY��HYΪǿ�ᣬ��Һ��pH=7������Һ��c��OH-��=c��H+����c��Na+��=c��Y-��������HY��Ũ��Ϊ0.1mol/L��
��pH=9��˵��NaZΪǿ�������Σ�HZΪ���ᣬ�������ӻ���������õ�ˮ���������������Ũ�ȣ�
��HZΪ���ᣬ��Һ��ֻ�ܲ��ֵ���������ӣ�����Һ��Ϻ����������Һ��ʾ���ԣ�
��2���ٸ������Ũ�Ⱥ���Һ��pH�ж����ǿ����
��K��ʱ����Һ��Ӧ�������Ϊ�����ʵ�����MOH��MA�����������غ�����жϣ�
���
�⣺��1���ٵ��������Ũ�ȵ�����Һ�к��е����ʵ�����HA���������ƣ�����HAΪ���ᣬ�����ʵ�����ȵ�HA���������ƻ�ϣ�����ǡ�÷�Ӧ����ǿ��������NaX����Ӧ�����Һ�ʼ��ԣ���Һ��pH��7��c��OH-����c��H+��������X-���Ӳ���ˮ�⣬��c��Na+����c��X-����������Һ������Ũ�ȴ�СΪ��c��Na+����c��X-����c��OH-����c��H+����
�ʴ�Ϊ������c��Na+����c��X-����c��OH-����c��H+����
�������е������HY��NaOH��Һ��Ӧ����NaY������HYΪǿ�ᣬ��Ӧ�����ҺpH=7������Һ��c��OH-��=c��H+�������ݵ���غ�ɵ�c��Na+��=c��Y-��������HY��Ũ��=c��NaOH��=0.1mol/L��HY����ҺpH=1��
�ʴ�Ϊ��1��
��pH=9��˵��NaZΪǿ�������Σ�HZΪ���ᣬ��HZ��NaOH������Ӧ�����ӷ���ʽΪ��HZ+OH-�TH2O+Z-��������Һ����ˮ�������c��OH-��=
mol/L=1��10-5mol/L��
�ʴ�Ϊ��HZ+OH-=H2O+Z-��1��10-5��
�ܸ��ݢۿ�֪HZΪ���ᣬpH=2����Һ��������Ũ��Ϊ0.01mol/L����HZ��Ũ�ȴ���0.01mol/L����pH=12������������Һ���������Ƶ�Ũ��Ϊ0.01mol/L������Һ��Ϻ����������Һ��ʾ���ԣ���Һ��pH=b��7��
�ʴ�Ϊ������
��1���٢ٸ���ͼ��֪��0.01mol?L-1HA��Һ��pH=2��������Ũ�ȵ�����Ũ�ȣ����Ը�����ǿ�ᣬ
�ʴ�Ϊ��ǿ��0.01 mol/L HA��pH=2��
��K��ʱ�������MOH�����ʵ���=0.02mol?L-1��0.1L=0.002mol������HA�����ʵ���Ϊ��0.01mol?L-1��0.1L=0.001mol�����������غ�ɵã�c��M+��+c��MOH��=2c��A-����
�ʴ�Ϊ��=��
�ʴ�Ϊ������c��Na+����c��X-����c��OH-����c��H+����
�������е������HY��NaOH��Һ��Ӧ����NaY������HYΪǿ�ᣬ��Ӧ�����ҺpH=7������Һ��c��OH-��=c��H+�������ݵ���غ�ɵ�c��Na+��=c��Y-��������HY��Ũ��=c��NaOH��=0.1mol/L��HY����ҺpH=1��
�ʴ�Ϊ��1��
��pH=9��˵��NaZΪǿ�������Σ�HZΪ���ᣬ��HZ��NaOH������Ӧ�����ӷ���ʽΪ��HZ+OH-�TH2O+Z-��������Һ����ˮ�������c��OH-��=
| 10-14 |
| 10-9 |
�ʴ�Ϊ��HZ+OH-=H2O+Z-��1��10-5��
�ܸ��ݢۿ�֪HZΪ���ᣬpH=2����Һ��������Ũ��Ϊ0.01mol/L����HZ��Ũ�ȴ���0.01mol/L����pH=12������������Һ���������Ƶ�Ũ��Ϊ0.01mol/L������Һ��Ϻ����������Һ��ʾ���ԣ���Һ��pH=b��7��
�ʴ�Ϊ������
��1���٢ٸ���ͼ��֪��0.01mol?L-1HA��Һ��pH=2��������Ũ�ȵ�����Ũ�ȣ����Ը�����ǿ�ᣬ
�ʴ�Ϊ��ǿ��0.01 mol/L HA��pH=2��
��K��ʱ�������MOH�����ʵ���=0.02mol?L-1��0.1L=0.002mol������HA�����ʵ���Ϊ��0.01mol?L-1��0.1L=0.001mol�����������غ�ɵã�c��M+��+c��MOH��=2c��A-����
�ʴ�Ϊ��=��
���������⿼������Һ���������ҺpH�ļ��㡢�ε�ˮ��ԭ����Ӧ�á�����Ũ�ȴ�С�Ƚϵȣ���Ŀ�����֪ʶ��϶࣬ע��������Һ���������Һ��pH�Ĺ�ϵ���ܹ����ݵ���غ㡢�ε�ˮ��ԭ���������غ��ж���Һ������Ũ�ȴ�С����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 ��ĩ���ƾ�ϵ�д�
��ĩ���ƾ�ϵ�д� ���ɿ��ñ���ϵ�д�
���ɿ��ñ���ϵ�д�
�����Ŀ
��������Һ�У�һ���ܴ���������������ǣ�������
A��������
| ||
| B�����д���Al3+����Һ�У�SO42-��S2-��AlO2-��ClO- | ||
| C����ʹpH��ֽ����ɫ����Һ�У�K+��Ba2+��Cl-��Br- | ||
| D�������£�����ˮ�������c��H+��=1��10-13mol?L-1����Һ�У�K+��Fe3+��Cl-��SO42- |
����Ԫ���У���������Ԫ�ص��ǣ�������
| A���� | B���� | C��ͭ | D���� |
���꣬��ѧ���˹��ϳ���λ��Ԫ�����ڱ��е������ڵڢ�A������ֺ���
Uus��
Uus������˵������ȷ���ǣ�������
293 117 |
294 117 |
A��
| ||||
B��
| ||||
C��
| ||||
D��
|
���и���ԭ��������ʾ������Ԫ���У��ܹ��γɹ��ۻ�������ǣ�������
| A��11��17 | B��11��10 |
| C��1��17 | D��1��8 |
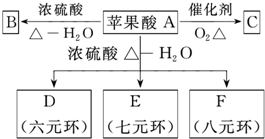 ƻ���ף�ACV����һ����ƻ�����Ͷ��ɵ�������Ʒ�����нⶾ����֬��ҩЧ����Ҫ��������Ϊƻ���ᣮ��ƻ�����ڷ����ᴿ��Ļ�ѧ�������£�
ƻ���ף�ACV����һ����ƻ�����Ͷ��ɵ�������Ʒ�����нⶾ����֬��ҩЧ����Ҫ��������Ϊƻ���ᣮ��ƻ�����ڷ����ᴿ��Ļ�ѧ�������£� SO2�dz����Ĵ�����Ⱦ��֮һ���ҹ��涨������SO2�������ó���0.02mg/L��
SO2�dz����Ĵ�����Ⱦ��֮һ���ҹ��涨������SO2�������ó���0.02mg/L��