��Ŀ����
����Ŀ�����볣��(Ka��Kb)���ܶȻ�����(Ksp)���ж��������ʵ���Ҫ���������й�����Щ�����ļ����������ȷ���ǣ� ��
A. ij��������ҺpH=4.3�������Զ������룬����һ������ƽ�ⳣ��K1=1.0��10-8.60
B. Ka(HCN)<Ka(CH3COOH)��˵��ͬŨ��ʱ����������Աȴ���ǿ
C. Ksp(AgI)<Ksp(AgCl)����AgCl����Һ�м���KI��Һ����ֻ�ɫ����
D. Ksp(AgCl)<Ksp(AgOH)��˵��AgOH��AgCl��������ϡ����
���𰸡�C
��������A. ij��������ҺpH=4.3�������Զ������룬����һ������ƽ�ⳣ��K1=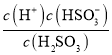 =
=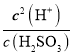 =
=![]() ��1.0��10-8.60����A����B. ���볣��ԽС������Խ����Ka(HCN)<Ka(CH3COOH)��˵��ͬŨ��ʱ����������Աȴ���������B����C. Ksp(AgI)<Ksp(AgCl)����AgCl����Һ�м���KI��Һ����ֻ�ɫ����AgI����C��ȷ��D. Ksp(AgCl)<Ksp(AgOH)��ֻ��˵�����ǵ��ܶȻ���С��ϵ������˵��AgOH��AgCl��������ϡ���ᣬ��D����ѡC��
��1.0��10-8.60����A����B. ���볣��ԽС������Խ����Ka(HCN)<Ka(CH3COOH)��˵��ͬŨ��ʱ����������Աȴ���������B����C. Ksp(AgI)<Ksp(AgCl)����AgCl����Һ�м���KI��Һ����ֻ�ɫ����AgI����C��ȷ��D. Ksp(AgCl)<Ksp(AgOH)��ֻ��˵�����ǵ��ܶȻ���С��ϵ������˵��AgOH��AgCl��������ϡ���ᣬ��D����ѡC��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ