��Ŀ����
��6�֣���ͼ��ʾ�����رշ���Kʱ������г���1.5 mol A��3.5 mol B�������г��� 3 mol A��7 mol B����ʼʱ���ס��������ΪV L������ͬ�¶Ⱥ��д������ڵ������£��������и��Է������з�Ӧ��3A(g)��2B(g) C(g)
C(g) ��2D(g)����H<0
��2D(g)����H<0
�ﵽƽ�⣨��ʱ��V(��)=0.86V L��

��ش�
��1������B��ת����Ϊ ��
��2������D������C�����ʵ����Ƚϣ� �����ȡ�����ǰ�ߴ������ߴ���
��3����K����һ��ʱ�����´�ƽ�⣨��ʱ���ҵ����Ϊ ���ú�V�Ĵ���ʽ��ʾ����ͨ��������������Բ��ơ���
 C(g)
C(g) ��2D(g)����H<0
��2D(g)����H<0�ﵽƽ�⣨��ʱ��V(��)=0.86V L��

��ش�
��1������B��ת����Ϊ ��
��2������D������C�����ʵ����Ƚϣ� �����ȡ�����ǰ�ߴ������ߴ���
��3����K����һ��ʱ�����´�ƽ�⣨��ʱ���ҵ����Ϊ ���ú�V�Ĵ���ʽ��ʾ����ͨ��������������Բ��ơ���
��1��20% ��2�����ߴ� ��3��0.72V
(1) 3A(g)��2B(g) C(g)
C(g) ��2D(g)
��2D(g)
��ʼ����mol�� 3 7 0 0
ת������mol�� 3x 2x x 2x
ƽ������mol�� 3��3x 7��2x x 2x
��
���2x��1.4
����Bת������1.4��7��100����20��
��2�����DZ��ֵ��µ��ݵģ������DZ��ֵ��µ�ѹ�ġ�����Ϊ��Ӧ�������С�Ŀ��淴Ӧ���������з�Ӧ���ת���ʸ��ڼ��з�Ӧ���ת���ʣ��������C�����ʵ����ࡣ
��3����K�����൱���ǵ��µ�ѹ�µĿ��淴Ӧ����˺�ԭ������ƽ���ǵ�Ч�ġ���ʱ����B��10.5mol��0.2��2.1mol��������A��2.1mol��2��3��3.15mol������C��D�ֱ���1.05mol��2.1mol�������ƽ��ʱ�������������X������
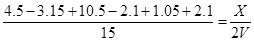
���X��1.72VL
�����ҵ����Ϊ1.72VL��VL��0.72V��
 C(g)
C(g) ��2D(g)
��2D(g)��ʼ����mol�� 3 7 0 0
ת������mol�� 3x 2x x 2x
ƽ������mol�� 3��3x 7��2x x 2x
��

���2x��1.4
����Bת������1.4��7��100����20��
��2�����DZ��ֵ��µ��ݵģ������DZ��ֵ��µ�ѹ�ġ�����Ϊ��Ӧ�������С�Ŀ��淴Ӧ���������з�Ӧ���ת���ʸ��ڼ��з�Ӧ���ת���ʣ��������C�����ʵ����ࡣ
��3����K�����൱���ǵ��µ�ѹ�µĿ��淴Ӧ����˺�ԭ������ƽ���ǵ�Ч�ġ���ʱ����B��10.5mol��0.2��2.1mol��������A��2.1mol��2��3��3.15mol������C��D�ֱ���1.05mol��2.1mol�������ƽ��ʱ�������������X������
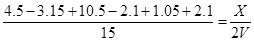
���X��1.72VL
�����ҵ����Ϊ1.72VL��VL��0.72V��

��ϰ��ϵ�д�
�����Ŀ
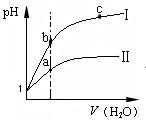
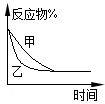
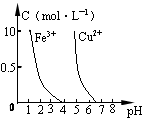
 3C(g)+D(s)��Ӱ�죬�ҵ�ѹǿ�ȼ�ѹǿ��
3C(g)+D(s)��Ӱ�죬�ҵ�ѹǿ�ȼ�ѹǿ��



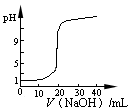
 2CO�ﵽƽ���,ƽ�������к�14C��������
2CO�ﵽƽ���,ƽ�������к�14C��������  b Z(g)����Ӧ�ﵽƽ����X��ת����Ϊ25%�����ң���ͬ��ͬѹ�»���÷�Ӧǰ���������ܶ��Ƿ�Ӧ���������ܶȵ�5/6����a��b����ֵ������( B)
b Z(g)����Ӧ�ﵽƽ����X��ת����Ϊ25%�����ң���ͬ��ͬѹ�»���÷�Ӧǰ���������ܶ��Ƿ�Ӧ���������ܶȵ�5/6����a��b����ֵ������( B)