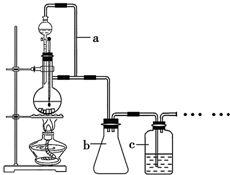
��Ŀ����
13�� ij��ȤС��̽��SO2���廹ԭFe3+��I2������ʹ�õ�ҩƷ��װ����ͼ��ʾ��
ij��ȤС��̽��SO2���廹ԭFe3+��I2������ʹ�õ�ҩƷ��װ����ͼ��ʾ����1��SO2���廹ԭFe3+��Ӧ�IJ�����Fe2+��SO42-�������ӷ��ţ���
��2������ʵ�鷽������������ʵ������ȡ����SO2����BD��
A��Na2SO3��Һ��HNO3����������B��Na2SO3������70%Ũ����
C���������ڴ�����ȼ�ա�������D��ͭ��ŨH2SO4����
��3��װ��C�������dz�ȥ�����SO2����ֹ��Ⱦ������
��4��������װ����ͨ�������SO2��Ϊ����֤A��SO2��Fe3+������������ԭ��Ӧ������ȡA�е���Һ���ֳ����ݣ������������ʵ�飺
�����٣�����һ����Һ�м���KMnO4��Һ���Ϻ�ɫ��ȥ��
�����ڣ�����һ����Һ����KSCN��Һ������죬�ټ������Ƶ���ˮ����Һ��죮
�����ۣ�����������Һ������ϡ�����ữ��BaCl2��������ɫ������
�����������������Ƿ����٣�ԭ������ΪA����Һ�к���SO2��SO2Ҳ��ʹKMnO4��Һ��ɫ��
��5���ܱ���I-�Ļ�ԭ������SO2��������װ��B����Һ��ɫ��ȥ��д���й����ӷ���ʽ��I2+SO2+2H2O=4H++2I-+SO42-��
���� ��1������װ��A��Ӧ�����ӷ���ʽSO2+2Fe3++2H2O�T2Fe2++SO42-+4H+���н��
��2��ʵ������ȡ����Ҫ���Dz������㡢���ơ����ܺ����ʣ�
��3������������д̼�����ζ����Ⱦ������������������Һ���ն�������ֹ������Ⱦ��
��4�������������������ط���������ԭ��Ӧʹ���������Һ��ɫ��Fe2+Ҳʹ���������Һ��ɫ��
��5������������ʹ���е�ĵ�����Һ��ɫ��˵��������������ԭ��Ӧ�����ݻ�ԭ���Ļ�ԭ�Դ��ڻ�ԭ����Ļ�ԭ�Է�����
��� �⣺��1�����������������ӷ�Ӧ�����ӷ���ʽΪ2Fe3++SO2+2H2O=2Fe2++SO42-+4H+����Ӧ�������ӱ��������������ӣ����Ի�ԭ����Ϊ�������ӣ�����������SO42-��SO2���廹ԭFe3+��Ӧ�IJ�����Fe2+��SO42-���ʴ�Ϊ��Fe2+��SO42-��
��2��A���������ǿ�����ԣ��ܹ����������������������ƣ����õ������������壬��A����
B��Ũ���������ǿ���ԣ���Ũ����ӷ�������������Һ��Ũ�����ܹ���Ӧ���ɶ����������壬��B��ȷ��
C���������ڴ�����ȼ�գ������������ƣ�������ô����Ķ�������C����
D��Cu��Ũ�����ڼ������������ɶ�����������ͭ��ˮ����֪�Ʊ���������D��ȷ��
�ʴ�Ϊ��BD��
��3����������������������������д̼�����ζ��ֱ���ŷŻ���Ⱦ���������ڶ��������ܺͼӦ�����κ�ˮ�����ü�Һ����������������װ��C������Ϊ������SO2β������ֹ��Ⱦ������
�ʴ�Ϊ����ȥ�����SO2����ֹ��Ⱦ������
��4�����������л�ԭ�ԣ����������ǿ�����ԣ������������������ط���������ԭ��Ӧʹ���������Һ��ɫ��Fe2+Ҳʹ���������Һ��ɫ�����Է����ٲ�������
�ʴ�Ϊ�������٣���ΪA����Һ�к���SO2��SO2Ҳ��ʹKMnO4��Һ��ɫ��
��5������������ʹ���е�ĵ�����Һ��ɫ��˵��������������ԭ��Ӧ�����������������������ǻ�ԭ������ԭ���Ļ�ԭ�Դ��ڻ�ԭ����Ļ�ԭ�ԣ��䷴Ӧ�����ӷ���ʽΪ��I2+SO2+2H2O=4H++2I-+SO42-��
�ʴ�Ϊ��װ��B����Һ��ɫ��ȥ��I2+SO2+2H2O=4H++2I-+SO42-��
���� ���⿼������������ʵ�鷽������ƣ���Ŀ�Ѷ��еȣ������漰�����Ի�ԭ��ǿ���Ƚϡ�����ʵ�鷽������������۵�֪ʶ����ȷ����Ũ��������ʡ���������ļ��鷽����֪ʶΪ�����ؼ���

 ���100��1�ž�ϵ�д�
���100��1�ž�ϵ�д�| A�� | N P Cl | B�� | P O S | C�� | N O S | D�� | O P Cl |
| A�� | 2Na2O2+2CO2�T2Na2CO3+O2 | |
| B�� | Ca��O H��2+2NH4Cl=CaCl2+2NH3��+2H2O | |
| C�� | Fe2O3+3CO $\frac{\underline{\;����\;}}{\;}$2Fe+3CO2 | |
| D�� | Cu+2H2SO4��Ũ��$\frac{\underline{\;����\;}}{\;}$CuSO4+SO2��+2H2O |
| A�� | �������뵥����Ϊͬ�������� | |
| B�� | 1mol���������밢��٤��������ˮ����������ԭ������� | |
| C�� | ���ַ��ӵ�Ħ������Ϊ192g | |
| D�� | �ڱ�״���£�1mol�������ʵ����Ϊ22.4L�� |
| A�� | �����ؽ������ӵķ�ˮ���������� | B�� | ��Cu2+��Fe3+�ķ�ˮ�ó��������� | ||
| C�� | ����ϸ������ˮ�ó���ɱ�� | D�� | ����������Է�ˮ���кͷ����� |
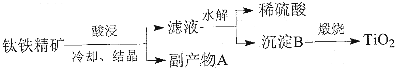
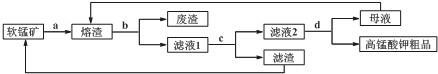
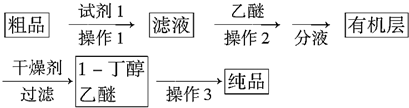
 ijͬѧ�������Ϻ������һ��1-�����ĺϳ�·�ߣ�
ijͬѧ�������Ϻ������һ��1-�����ĺϳ�·�ߣ�