��Ŀ����
11��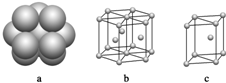 A��B��C��D��E��F��G��ԭ�������������������Ԫ�أ�����A��B��C��D��EΪ������Ԫ�أ�F��GΪ��������Ԫ�أ���֪��A��ԭ�Ӱ뾶��С��Ԫ�أ�B��C��D�ǽ��ڵ��������Ԫ�أ�C�����������Ӻ�E�Ķ��������Ӿ�����ͬ�ĵ��Ӳ�ṹ��FԪ�صĻ�̬ԭ�Ӿ��������ɵ����ӣ�G�Ǣ�B���Ԫ�أ��ش��������⣺
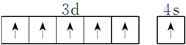
A��B��C��D��E��F��G��ԭ�������������������Ԫ�أ�����A��B��C��D��EΪ������Ԫ�أ�F��GΪ��������Ԫ�أ���֪��A��ԭ�Ӱ뾶��С��Ԫ�أ�B��C��D�ǽ��ڵ��������Ԫ�أ�C�����������Ӻ�E�Ķ��������Ӿ�����ͬ�ĵ��Ӳ�ṹ��FԪ�صĻ�̬ԭ�Ӿ��������ɵ����ӣ�G�Ǣ�B���Ԫ�أ��ش��������⣺��1��FԪ�ص�ԭ�ӻ�̬�۲�����Ų�ͼ��
 ��B��C��DԪ�صĵ�һ�������ɴ�С��˳����N��O��C����Ԫ�ط��ű�ʾ����
��B��C��DԪ�صĵ�һ�������ɴ�С��˳����N��O��C����Ԫ�ط��ű�ʾ������2����Ԫ��B��A�γɵ�ˮ����������廯�����У�Ԫ��B���ӻ���ʽΪsp2�ӻ���Ԫ��A��C�γɵ����X���ӵĿռ乹��Ϊ�����Σ���������Xͨ�뺬��Ԫ��G����ɫ��������Һ�У���Ӧ�����ӷ���ʽΪCu2++4NH3=[Cu��NH3��4]2+��
��3�����³�ѹ�£���23gҺ̬������B2A6D��������D�ĵ��ʳ�ַ�Ӧ������BD2�����A2DҺ�壬ͬʱ�ų�683.5kJ���������÷�Ӧ���Ȼ�ѧ����ʽΪ��C2H6O��1��+��g��=2CO2��g��+3H2O��1����H=-1367.0 kJ•mol-1��
��4��E���ʾ�����ԭ�ӵĶѻ�ģ����ͼ��������ͼ�е�c����λ����12��
���� A��B��C��D��E��F��G��ԭ�������������������Ԫ�أ�����A��EΪ����������Ԫ�أ�A��ԭ�Ӱ뾶��С��Ԫ�أ���AΪHԪ�أ�C�����������Ӻ�E�Ķ��������Ӿ�����ͬ�ĵ��Ӳ�ṹ��C�ڵڶ����ڵڢ�A�壬E�ڵ������ڵڢ�A�壬����C��NԪ�أ�E��MgԪ�أ�B��C��D�ǽ��ڵ��������Ԫ�أ�B��C��Dԭ������������������B��̼Ԫ�أ�D��OԪ�أ�F��GΪ��������Ԫ�أ�F�Ļ�̬ԭ�Ӿ��������ɵ����ӣ���F�ļ۵����Ų�Ϊ3d54s1����24��Ԫ�أ���FΪCrԪ�أ�G�Ǣ�B���Ԫ�أ�����G��CuԪ�أ��ݴ˽��
��� �⣺A��B��C��D��E��F��G��ԭ�������������������Ԫ�أ�����A��EΪ����������Ԫ�أ�A��ԭ�Ӱ뾶��С��Ԫ�أ���AΪHԪ�أ�C�����������Ӻ�E�Ķ��������Ӿ�����ͬ�ĵ��Ӳ�ṹ��C�ڵڶ����ڵڢ�A�壬E�ڵ������ڵڢ�A�壬����C��NԪ�أ�E��MgԪ�أ�B��C��D�ǽ��ڵ��������Ԫ�أ�B��C��Dԭ������������������B��̼Ԫ�أ�D��OԪ�أ�F��GΪ��������Ԫ�أ�F�Ļ�̬ԭ�Ӿ��������ɵ����ӣ���F�ļ۵����Ų�Ϊ3d54s1����24��Ԫ�أ���FΪCrԪ�أ�G�Ǣ�B���Ԫ�أ�����G��CuԪ�أ�
��1��FԪ�ص�ԭ�ӻ�̬�۲�����Ų�ͼ�ǣ� ��ͬ������ԭ����������Ԫ�ص�һ�����ܳ��������ƣ���NԪ��ԭ��2p�ܼ�����3�����ӣ�Ϊ�����ȶ�״̬�������ϵͣ���һ�����ܸ���ͬ��������Ԫ�أ��ʵ�һ�������ɴ�С��˳��ΪN��O��C��
��ͬ������ԭ����������Ԫ�ص�һ�����ܳ��������ƣ���NԪ��ԭ��2p�ܼ�����3�����ӣ�Ϊ�����ȶ�״̬�������ϵͣ���һ�����ܸ���ͬ��������Ԫ�أ��ʵ�һ�������ɴ�С��˳��ΪN��O��C��
�ʴ�Ϊ�� ��N��O��C��
��N��O��C��
��2����Ԫ��B��A�γɵ�ˮ����������廯����ΪC2H4��������Cԭ���γ�3���Ҽ���û�йµ��Ӷԣ���Cԭ�Ӳ�ȡsp2�ӻ���NH3���ӵĿռ����幹��Ϊ�������Σ�����ͭ�������NH3ˮ��Һ��Ӧ�õ��������ӵ�����ɫ��Һ���������ӵĻ�ѧʽΪ[Cu��NH3��4]2+����Ӧ���ӷ���ʽΪ��Cu2++4NH3=[Cu��NH3��4]2+��
�ʴ�Ϊ��[Cu��NH3��4]2+��Cu2++4NH3=[Cu��NH3��4]2+��
��3�����³�ѹ�£���23gҺ̬������C2H6O��������O2��ַ�Ӧ������CO2�����H2OҺ�壬ͬʱ�ų�683.5kJ��������C2H6O�����ʵ���Ϊ$\frac{23g}{46g/mol}$=0.5mol����1molC2H6O��Ӧ�ų�����Ϊ683.5kJ��$\frac{1mol}{0.5mol}$=1367.0 kJ���÷�Ӧ���Ȼ�ѧ����ʽΪ��C2H6O��1��+��g��=2CO2��g��+3H2O��1����H=-1367.0 kJ•mol-1��
�ʴ�Ϊ��C2H6O��1��+��g��=2CO2��g��+3H2O��1����H=-1367.0 kJ•mol-1��
��4��Mg���ʾ������������ܶѻ���������ͼ�е�c����λ����12��
�ʴ�Ϊ��c��12��
���� �����Ƕ����ʽṹ�Ŀ��飬�漰��������Ų��������ܡ��ӻ���ʽ�����ӿռ乹�͡����������ṹ�����㡢�Ȼ�ѧ����ʽ��д�ȣ��ƶ�Ԫ���ǽ���Ĺؼ�����Ҫѧ���߱���ʵ�Ļ����Ѷ��еȣ�

 �»����ܶ�Ա��ϵ�д�
�»����ܶ�Ա��ϵ�д� ����ͼ����ּ��������ҵ֣�ݴ�ѧ������ϵ�д�
����ͼ����ּ��������ҵ֣�ݴ�ѧ������ϵ�д�| A�� | 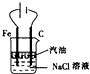 ����ͼװ����ȡFe��OH��2���� | |
| B�� | 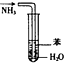 ����ͼװ������NH3������ֹ���� | |
| C�� |  ����ͼװ������AlCl3������Һ�Ʊ�AlCl3���� | |
| D�� | 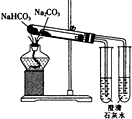 ����ͼװ�ñȽ�Na2CO2��NaHCO3���ȶ��� |
| A�� | ����ԭ�ӵĵ��Ӳ���ȫ������s���� | |
| B�� | ԭ�Ӻ����M���ϵ�s�ܼ���p�ܼ��������˵��ӣ���d�������δ���е��ӵ�����ԭ�� | |
| C�� | ���������Ų�ʽΪ2s22p6��ԭ�Ӻ����������Ų�ʽΪ2s22p6������ | |
| D�� | 3p�ܼ���ֻ��һ���չ����ԭ�Ӻ�3p�ܼ�����һ��δ�ɶԵ��ӵ�ԭ�� |

| A�� | 1 molʯī���ܼ��ܱ�1 mol���ʯ���ܼ���С1.9 kJ | |
| B�� | ʯī�ͽ��ʯ��ת���������仯 | |
| C�� | ���ʯ���ȶ���ǿ��ʯī | |
| D�� | C��s��ʯī���TC��s�����ʯ����H=+1.9 kJ•mol-1 |
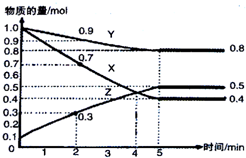 ij�¶�ʱ����2L������X��Y��Z������̬������ʱ��ı仯��ϵ������ͼ��ʾ������˵����ȷ���ǣ�������
ij�¶�ʱ����2L������X��Y��Z������̬������ʱ��ı仯��ϵ������ͼ��ʾ������˵����ȷ���ǣ�������| A�� | ��Ӧ��ʼ��5min����X��ʾ�ķ�Ӧ����Ϊ0.3mol•L-1•min-1 | |
| B�� | ��Ӧ��ʼ��2min����Z��ʾ�ķ�Ӧ����Ϊ0.05mol•L-1•min-1 | |
| C�� | ��Ӧ��ʼ��4min��Ӧ�ﵽƽ��״̬ | |
| D�� | ��Ӧ�Ļ�ѧ����ʽΪ��2X��g��+Y��g��?3Z��g�� |
| A�� | �������չ����У�Br2ֻ�������� | |
| B�� | �������չ��̶�������������ԭ��Ӧ | |
| C�� | �ô�����Һ�����ռ�ʱ����������ԭ�� | |
| D�� | �ô�����Һ�����ռ�ֻ�����˸��ֽⷴӦ |
| A�� | CH4 | B�� | C3H4 | C�� | C4H8 | D�� | C2H4 |
 �Ҵ���������ȩ��ʵ��װ����ͼ��ʾ���г������ͼ���������δ����������������������ǣ�������
�Ҵ���������ȩ��ʵ��װ����ͼ��ʾ���г������ͼ���������δ����������������������ǣ�������| A�� | a����ʢ�Ĺ��������CuO | |
| B�� | d���Ҵ����÷�ˮԡ���� | |
| C�� | c����֧�Թ�b������ɫҺ����� | |
| D�� | c����֧�Թ�b�ɻ��ɴ�������ͨ�Թ� |
