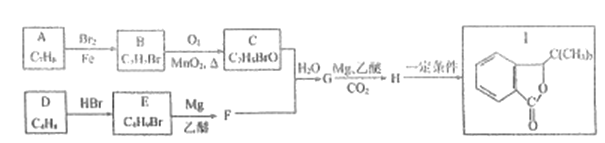
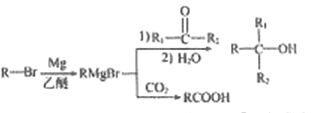
��Ŀ����
����Ŀ�����������������ճ�����������Ӧ�ù㷺�IJ��ϡ���ش���������:
��1����̬��ԭ�ӵļ۵����Ų�ͼΪ_______
��2����Ԫ�س�����������Fe2+��Fe3+,�ȶ���Fe2+_____Fe3+(��������������С����)��ԭ����____��
��3�������������ܴ�����ƽ���NH4ClO4�ķֽ⣬NH4+�ĽṹʽΪ______(�����λ��)�����е�ԭ�ӵ��ӻ���ʽΪ______;�� ClO4-��Ϊ�ȵ�����ķ��ӻ�����Ϊ_________(��д����)��
��4������������ԭ�Ӳ������������ѻ�����������Ŀռ�������Ϊ____ (�ú�����ʽ�ӱ�ʾ)��
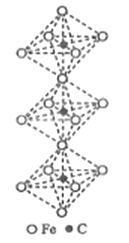
��5��ij�����������������ᄃ����ͼ��ʾ������A��B������ɡ���û�������Fe2+��Fe3+��O2-�ĸ�����Ϊ______(�����������)����֪�þ�����ܶ�dg/cm3,�����ӵ�������ֵΪNA��������Ϊ______nm(�ú�d��NA�Ĵ���ʽ��ʾ)��
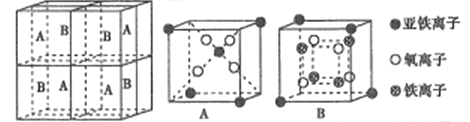
��6��һ������̼�γɵļ�϶������ľ���ṹ��ͼ��ʾ������̼ԭ��λ����ԭ���γɵİ���������ġ�ÿ����ԭ����Ϊ���������干�á���û�����Ļ�ѧʽΪ_______��
���𰸡�![]() С�� Fe2+�ļ۵����Ų�ʽΪ3d6,Fe3+�ļ۵����Ų�ʽΪ3d5,Fe3+��3d�ܼ�Ϊ�����״̬�����ȶ�
С�� Fe2+�ļ۵����Ų�ʽΪ3d6,Fe3+�ļ۵����Ų�ʽΪ3d5,Fe3+��3d�ܼ�Ϊ�����״̬�����ȶ� 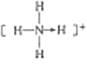 sp3 CCl4��PO43-
sp3 CCl4��PO43- ![]() 1:2:4
1:2:4  Fe3C
Fe3C
��������
������1������26��Ԫ�أ�3d��4s����ĵ���Ϊ�۵�������2���ܼ�ȫ�������������ȫ��ʱ�ȶ�����3��NH4+����������ṹ��Nԭ�Ӽ۵��Ӷ�����![]() ��ԭ������ͬ���۵�����Ҳ��ͬ�ķ��ӻ����ӻ�Ϊ�ȵ���������4������Ŀռ�������Ϊ������ԭ�����
��ԭ������ͬ���۵�����Ҳ��ͬ�ķ��ӻ����ӻ�Ϊ�ȵ���������4������Ŀռ�������Ϊ������ԭ�����![]() �����������5�����ݾ�̯ԭ�����Fe2+��Fe3+��O2-�ĸ�����������
�����������5�����ݾ�̯ԭ�����Fe2+��Fe3+��O2-�ĸ�����������![]() ���㾧���߳�����6�����ݾ�̯ԭ����㻯ѧʽ��
���㾧���߳�����6�����ݾ�̯ԭ����㻯ѧʽ��
��������1������26��Ԫ�أ��۵����Ų�ͼΪ![]() ����2��Fe2+�ļ۵����Ų�ʽΪ3d6��Fe3+�ļ۵����Ų�ʽΪ3d5��Fe3+��3d�ܼ�Ϊ�����״̬�����ȶ��������ȶ���Fe2+С��Fe3+����3��NH4+Ϊ��������ṹ���ṹʽΪ
����2��Fe2+�ļ۵����Ų�ʽΪ3d6��Fe3+�ļ۵����Ų�ʽΪ3d5��Fe3+��3d�ܼ�Ϊ�����״̬�����ȶ��������ȶ���Fe2+С��Fe3+����3��NH4+Ϊ��������ṹ���ṹʽΪ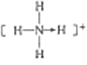 ��Nԭ�Ӽ۵��Ӷ�����
��Nԭ�Ӽ۵��Ӷ�����![]() ����ԭ�ӵ��ӻ���ʽΪsp3���� ClO4-��Ϊ�ȵ�����ķ��ӻ�����ΪCCl4��PO43-����4��������ԭ�Ӳ������������ѻ�������1������������ԭ����
����ԭ�ӵ��ӻ���ʽΪsp3���� ClO4-��Ϊ�ȵ�����ķ��ӻ�����ΪCCl4��PO43-����4��������ԭ�Ӳ������������ѻ�������1������������ԭ����![]() ������ԭ�ӵİ뾶��a�����߳���
������ԭ�ӵİ뾶��a�����߳���![]() ������Ŀռ�������Ϊ
������Ŀռ�������Ϊ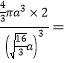
![]() ����5�����ݾ�̯ԭ����ÿ��A�к���Fe2+ -�ĸ���
����5�����ݾ�̯ԭ����ÿ��A�к���Fe2+ -�ĸ���![]() ����ÿ��A�к���O2-��4��ÿ��B�к���Fe2+ -�ĸ���
����ÿ��A�к���O2-��4��ÿ��B�к���Fe2+ -�ĸ���![]() ��ÿ��B�к���Fe3+ �ĸ���4����ÿ��A�к���O2-��4����������ÿ�����������ᄃ����4��A��4��B�����������������ÿ����������Fe2+��Fe3+��O2-�ĸ����ֱ���8��16��32����Ϊ1:2:4��������Ħ��������
��ÿ��B�к���Fe3+ �ĸ���4����ÿ��A�к���O2-��4����������ÿ�����������ᄃ����4��A��4��B�����������������ÿ����������Fe2+��Fe3+��O2-�ĸ����ֱ���8��16��32����Ϊ1:2:4��������Ħ��������![]() ���辧���߳���a nm����
���辧���߳���a nm����![]() ��a=
��a= ����6�����ݾ�̯ԭ��ÿ����ԭ����Ϊ���������干������������̼ԭ��������
����6�����ݾ�̯ԭ��ÿ����ԭ����Ϊ���������干������������̼ԭ��������![]() �����Ի�ѧʽ��Fe3C��
�����Ի�ѧʽ��Fe3C��

 ���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д�
���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д�����Ŀ����8�֣�.������(Si3N4)��һ�ָ����մɲ��ϣ�����Ӳ�ȴ��۵�ߡ���ѧ�����ȶ�����ҵ���ձ���øߴ����봿����1300����Ӧ��á�
��1���������ʣ��Ʋ�����մɵ���;�� ��
A�������ֻ�ҶƬ | B������ɫ���� | C����������ģ�� | D��������ͻ� |
��2���������մɿ���ʴ����ǿ����������⣬�������������ᷴӦ�����Ʋ���մɱ�����ḯʴ�Ļ�ѧ����ʽΪ ��
��3���������Ȼ���͵����������м�ǿ�ȷ�����Ӧ�����Ƶøߴ��ȵ����裬��Ӧ�Ļ�ѧ����ʽΪ ��