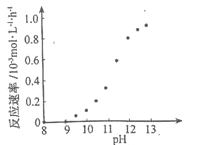
��Ŀ����
����Ŀ����ͼ�������Լ�ƿ��ǩ�ϵ����ݣ�
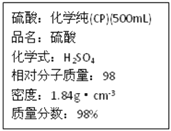
��1������������ʵ���Ũ��Ϊ_____________mol/L��
��2��ij��ѧС�������������ʵ��̽��ʱ����Ҫ240 mL4.6 mol/L��ϡ���ᣬ����Ҫȡ������Ũ����______mL��
��3��������4.6 mol/Lϡ����Ĺ����У����������������������Һ���ʵ���Ũ���к�Ӱ�죿(����ƫ������ƫ����������Ӱ����)
��δ����ȴ���Ƚ���Һע������ƿ��_______��
������ʱ���ӿ̶���_______��
������Ͳ��ȡŨ����ʱ����_______��
����Һʱ������������Һ��������ƿ����______��
��4��ʵ������г����������Ӧ��δ�����
��������ƿ�м�������ˮ����̶���1~2 cmʱ��Ӧ____________________________��
��������ˮʱ���������˿̶ȣ�Ӧ__________________��
���𰸡�18.462.5ƫ��ƫ��ƫ��ƫ���ý�ͷ�ιܵμ�����Һ��Һ�����ʹ��Ϳ̶���������������
��������
��1����ŨH2SO4�����ʵ���Ũ��=![]() mol/L=18.4mol/L��
mol/L=18.4mol/L��
��2��ʵ����û��240mL����ƿ��Ӧѡ��250mL����ƿ������ϡ�Ͷ��ɣ���ҪŨ��������=![]() =62.5mL��ϡ��Ũ����Ӧ��Ũ���������ڻ���ע��ˮ������ϵ��ò�����������Һ��
=62.5mL��ϡ��Ũ����Ӧ��Ũ���������ڻ���ע��ˮ������ϵ��ò�����������Һ��
��3������Һ���������������ʣ�δ����ȴ���Ƚ���Һע������ƿ�ж��ݣ���ȴ����Һ�����ƫС������������ҺŨ��ƫ�ߣ�
�ڶ���ʱ���ӹ۲�Һ�棬Һ���ٿ̶����Ϸ�����Һ���ƫ��������ҺŨ��ƫ����
������Ͳ��ȡŨ����ʱ���ӿ̶ȣ������Ķ���ƫ����ȡ��ʵ�����ƫС��Ҳ����˵��������������������Һ�����ʵ���Ũ�ȱ�ʵ��ƫ����
����Һʱ������������Һ��������ƿ���棬���²���������ģ����ʵ����ʵ���ƫС����ҺŨ��ƫ�ͣ�
��4���ٶ��ݵ���ȷ������������ƿ�м�������ˮ��Һ��������ƿ���̶�����1��2cmʱ��Ӧ���ý�ͷ�ιܵμ�����ˮ��Һ����̶������У�
�ڼ�����ˮʱ��������������ƿ���̶��ߣ�����ʵ��ʧ���������ȣ���Ҫ�������ơ�

 ��˼ά������ҵ���ټ��ִ�ѧ������ϵ�д�
��˼ά������ҵ���ټ��ִ�ѧ������ϵ�д�