��Ŀ����
ijС�������֪:������I >Fe3+>I2,3Br2+6FeCl2
>Fe3+>I2,3Br2+6FeCl2 2FeBr3+4FeCl3;I2+2S2
2FeBr3+4FeCl3;I2+2S2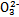
 S4
S4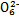 +2I-;CuI��һ�ְ�ɫ����(Ksp=1.3��1
+2I-;CuI��һ�ְ�ɫ����(Ksp=1.3��1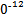 )��
)��
��.��С��Ϊȷ��һ�ݼӵ���(���ܺ���KIO3��KI��Mg2+��Fe3+)�ijɷ�,��ƶ���ʵ����Բ������֤��
(1)ʵ���������:
(2)�õ����п϶���������������������(�û�ѧʽ�����ӷ��ű���)��
(3)������Ϣ�ƶ�Fe3+��S4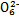 ��I2��Br2����������ǿ������˳������������������������
��I2��Br2����������ǿ������˳������������������������
(4)�ڢ۷���Һ�м�������KI�����,��Ӧ�����ӷ���ʽΪ��������������������������������������
��.�á���ӵ��������ⶨ����CuCl2��2H2O���������(��������I-������Ӧ������������)�Ĵ���,��������:
ȡ0.40 g��������ˮ,�������KI����,��ַ�Ӧ,���ɰ�ɫ����������������ζ�ָʾ��,��0.100 0 mol��L-1 Na2S2O3����Һ�ζ�,����ζ��յ�ʱ,����Na2S2O3����Һ20.00 mL��
(1)�ζ��յ������������
(2)CuCl2��Һ��KI��Ӧ�Ļ�ѧ����ʽΪ��������������������������������
(3)��������CuCl2��2H2O��������������������
 >Fe3+>I2,3Br2+6FeCl2
>Fe3+>I2,3Br2+6FeCl2 2FeBr3+4FeCl3;I2+2S2
2FeBr3+4FeCl3;I2+2S2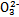
 S4
S4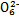 +2I-;CuI��һ�ְ�ɫ����(Ksp=1.3��1
+2I-;CuI��һ�ְ�ɫ����(Ksp=1.3��1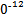 )��
)����.��С��Ϊȷ��һ�ݼӵ���(���ܺ���KIO3��KI��Mg2+��Fe3+)�ijɷ�,��ƶ���ʵ����Բ������֤��
(1)ʵ���������:
| ʵ�鲽�� | ʵ����̺����� | ��Ӧ���� | |
| ����1 | ȡһ��������,����������ˮ�ܽ�,����ϡ�����ữ,��������Һ��Ϊ3�� | _____________ | |
| �� �� 2 | �ڢٷ� ��Һ | 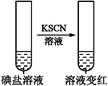 | �����п϶����������� |
| �ڢڷ� ��Һ | 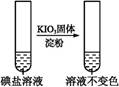 | _____________ | |
| �ڢ۷� ��Һ | 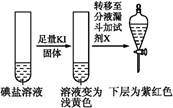 | XΪ������(�ѧʽ) | |
(2)�õ����п϶���������������������(�û�ѧʽ�����ӷ��ű���)��
(3)������Ϣ�ƶ�Fe3+��S4
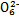 ��I2��Br2����������ǿ������˳������������������������
��I2��Br2����������ǿ������˳������������������������ (4)�ڢ۷���Һ�м�������KI�����,��Ӧ�����ӷ���ʽΪ��������������������������������������
��.�á���ӵ��������ⶨ����CuCl2��2H2O���������(��������I-������Ӧ������������)�Ĵ���,��������:
ȡ0.40 g��������ˮ,�������KI����,��ַ�Ӧ,���ɰ�ɫ����������������ζ�ָʾ��,��0.100 0 mol��L-1 Na2S2O3����Һ�ζ�,����ζ��յ�ʱ,����Na2S2O3����Һ20.00 mL��
(1)�ζ��յ������������
(2)CuCl2��Һ��KI��Ӧ�Ļ�ѧ����ʽΪ��������������������������������
(3)��������CuCl2��2H2O��������������������
��.(1)Fe3+��CCl4��(2)KI
(3)Br2>Fe3+>I2>S4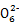
(4)2Fe3++2I- 2Fe2++I2,I
2Fe2++I2,I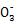 +5I-+6H+
+5I-+6H+ 3I2+3H2O
3I2+3H2O
��.(1)��ɫ��ȥ,����һ��ʱ��ָ�ԭɫ(����������ɫ���仯)
(2)2CuCl2+4KI 2CuI��+I2+4KCl
2CuI��+I2+4KCl
(3)85.5%
(3)Br2>Fe3+>I2>S4
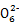
(4)2Fe3++2I-
 2Fe2++I2,I
2Fe2++I2,I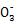 +5I-+6H+
+5I-+6H+ 3I2+3H2O
3I2+3H2O��.(1)��ɫ��ȥ,����һ��ʱ��ָ�ԭɫ(����������ɫ���仯)
(2)2CuCl2+4KI
 2CuI��+I2+4KCl
2CuI��+I2+4KCl(3)85.5%
��.(2)�ɲ���ڢۿ�֪��Һ��һ������KI,��ϼӵ�ʳ�ο�֪һ������KIO3;(4)��Һ�е�Fe3+��I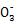 ����I-��Ӧ;��.����2CuCl2+4KI
����I-��Ӧ;��.����2CuCl2+4KI 2CuI��+I2+4KCl��I2+2S2
2CuI��+I2+4KCl��I2+2S2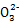
 S4
S4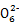 +2I-��֪CuCl2~S2
+2I-��֪CuCl2~S2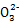 ,n(CuCl2)=n(S2
,n(CuCl2)=n(S2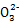 )=2��10-3 mol,m(CuCl2��2H2O)="0.342" g
)=2��10-3 mol,m(CuCl2��2H2O)="0.342" g
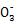 ����I-��Ӧ;��.����2CuCl2+4KI
����I-��Ӧ;��.����2CuCl2+4KI 2CuI��+I2+4KCl��I2+2S2
2CuI��+I2+4KCl��I2+2S2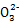
 S4
S4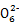 +2I-��֪CuCl2~S2
+2I-��֪CuCl2~S2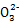 ,n(CuCl2)=n(S2
,n(CuCl2)=n(S2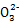 )=2��10-3 mol,m(CuCl2��2H2O)="0.342" g
)=2��10-3 mol,m(CuCl2��2H2O)="0.342" g
��ϰ��ϵ�д�
 ����ѧ���ʱѧ����ϵ�д�
����ѧ���ʱѧ����ϵ�д� �ʰ�Ӣ��ͬ����ϰ��ϵ�д�
�ʰ�Ӣ��ͬ����ϰ��ϵ�д�
�����Ŀ


