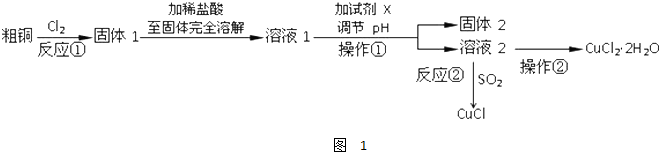
��Ŀ����
15����1���������ӵĴ�С��1-100nm ֮�䣬�����ЧӦ�����ڽ������ӶԹ��ɢ���γɵģ���2���������ʣ���Na ��Br2 ��Na2O ��NO2 ��CO2 ��SO3 ��NH3 ��H2S ��HCl ��H2SO4⑪Ba��OH��2⑫NaCl⑬����⑭NaCl��Һ��
���ڵ���ʵ��Ǣۢ���⑪⑫�����ڷǵ���ʵ��Ǣܢݢޢ�⑬���ܵ�����Ǣ�⑭��
��3��9.2g����������NOx�к���Nԭ����Ϊ0.2mol����NOx��Ħ������Ϊ46g/mol��x��ֵΪ2����Щ������NOx�ڱ�״���µ����ԼΪ4.48L��
���� ��1����������ֱ����1-100nm֮�䣬����Ķ�������������ڹⷢ��ɢ���γɵģ�
��2�����ݵ���ԭ����ʡ��ǵ���ʵĶ����жϣ�
�ܵ�������ʱ��뺬�����ɵ��ӻ��������ӣ�
����ʣ���ˮ��Һ�������״̬���ܵ���Ļ����
�ǵ���ʣ���ˮ��Һ�������״̬�¶����ܵ���Ļ����
��3����ԭ�����ʵ�����NOx����ȣ��ݴ˼���NOx�����ʵ������ٸ���M=$\frac{m}{n}$����NOx��Ħ����������������x��ֵ������9.2gNOx�����ʵ������Ӷ��������µ������
��� �⣺��1������ֱ������ 1��100nm֮�䣬С�ڿɼ���Ⲩ�����Թ�ɢ���γɶ����ЧӦ���ʴ�Ϊ��1-100nm�����ɢ�䣻
��2����Na���ڽ������ʣ��ܵ��粻�ǻ�����Ȳ��ǵ����Ҳ���Ƿǵ���ʣ� ��Br2 ���ܵ��磬���ڷǵ���ʵ��ʣ��Ȳ��ǵ����Ҳ���Ƿǵ���ʣ���Na2O���ܵ��磬����״̬���ܵ��磬���ڵ���ʣ� ��NO2 �������ܵ��룬���ڷǵ���ʣ���CO2 �������ܵ��룬���ڷǵ���ʣ���SO3 �������ܵ��룬���ڷǵ���ʣ���NH3 �������ܵ��룬���ڷǵ���ʣ���H2S���ܵ��磬�������ܵ��磬���ڵ���ʣ���HCl���ܵ��磬����ˮ�ܵ��磬���ڵ���ʣ� ��H2SO4���ܵ��磬����ˮ�ܵ��磬���ڵ���ʣ�⑪Ba��OH��2���ܵ��磬����ˮ������״̬���ܵ��磬���ڵ���ʣ�⑫NaCl���ܵ��磬����ˮ������״̬���ܵ��磬���ڵ���ʣ�⑬���Dz��ܵ��磬���ڷǵ���ʣ�⑭NaCl��Һ���ܵ��磬���ڻ����Ȳ��ǵ����Ҳ���Ƿǵ���ʣ��ʴ�Ϊ���ۢ���⑪⑫���ܢݢޢ�⑬����⑭��
��3�����ڵ�ԭ�����ʵ�����NOx����ȣ�9.2g����������NOx�к���Nԭ����Ϊ0.2mol����NOx�����ʵ���Ϊ0.2mol����NOx��Ħ������M=$\frac{m}{n}=\frac{9.2g}{0.2mol}$=46g/mol��
����14+16X=46�����X=2����Щ������NOx�����ʵ���Ϊ0.2mol���ڱ���µ����V=nVm=0.2mol��22.4L/mol=4.48L���ʴ�Ϊ��46g/mol��2��4.48��
���� ������Ҫ���齺������ʡ�����ʺͷǵ���ʣ���ȷ�����Ҫ�㼴�ɽ��ץס�������������ǽ��Ĺؼ�����ע����ԭ�������

| A�� | HCO3- | B�� | Al3+ | C�� | CH3COO- | D�� | H2PO4- |
| A�� | sp�ӻ� | B�� | sp2�ӻ� | C�� | sp3�ӻ� | D�� | sp4�ӻ� |
| A�� | ClO3-�Ŀռ乹��Ϊƽ�������� | |
| B�� | SiF4 �� SO32-������ԭ�Ӿ�Ϊ sp3 �ӻ� | |
| C�� | �����е�Ԫ���У����ĵ�һ��������� | |
| D�� | C2H5OH �����й����� 8 �����Լ���1 �� �� �� |
| A�� | Al2O3��Al��OH��3 | B�� | Fe��FeCl2 | C�� | S��SO2 | D�� | Na��Na2O |
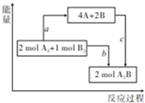 ��֪25�桢101kPa�£��Ͽ�1molA-A��1molB-B��1molA-B��ʱ��Ҫ����436kJ��498kJ��463kJ����������ͼ����ͬ�����£���Ӧ2A2+B2$\frac{\underline{\;��ȼ\;}}{\;}$2A2B��������ϵͼ������˵���в�����ǣ�������
��֪25�桢101kPa�£��Ͽ�1molA-A��1molB-B��1molA-B��ʱ��Ҫ����436kJ��498kJ��463kJ����������ͼ����ͬ�����£���Ӧ2A2+B2$\frac{\underline{\;��ȼ\;}}{\;}$2A2B��������ϵͼ������˵���в�����ǣ�������| A�� | a=1370kJ | |
| B�� | �÷�Ӧ�����ȷ�Ӧ | |
| C�� | A��Bԭ�Ӹ��������A2B | |
| D�� | ��Ӧ������ת����ʽ���ɻ�ѧ��ת��Ϊ���� |
| A�� | 17 g�������е�ԭ������ΪNA | |
| B�� | 2.4 g����þ��������ȫ��Ӧʱʧȥ�ĵ�����Ϊ0.1 NA | |
| C�� | ���³�ѹ�£�6.4 g��������������������ԭ����Ϊ0.2 NA | |
| D�� | ���³�ѹ�£�11.2 L�������еķ�����ΪNA |