��Ŀ����
 ���أ�
���أ�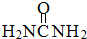 ������ɰ��Na2B4O7���ڸ��¸�ѹ�·�Ӧ���Ի�������
������ɰ��Na2B4O7���ڸ��¸�ѹ�·�Ӧ���Ի�������Na2B4O7+2CO��NH2��2�T4��BN��+Na2O+2CO2��+4H2O
��1��������Ӧ���к���һ��Ԫ�أ����̬ԭ�Ӿ���4�ֲ�ͬ�������ӣ�д���û�̬ԭ�ӵĵ����Ų�ʽ
��2��Ԫ��B��C��O��N��һ�������ɴ�С��˳��
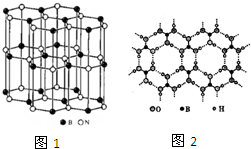
��3��ij������Ľṹ��ʯī���ƣ���ͼ1�����׳ơ���ʯī����
�پ�����B��Nԭ�ӵ��ӻ���ʽ�ֱ���
�ڰ�ʯī���ܵ��磬ԭ����B��Nԭ��֮��Ħм����ӱ�
��д������ʯī����һ����;
��4�����ؿ��������л����ʣ���Ҫ������[Fe��H2NCONH2��6]��NO3��3[�����������غ�������]���ṹ�ⶨ֪��1mol��������к���6NA����λ�����γ���λ��ʱ���ṩ�¶Ե��ӵ�ԭ����
��5������ɰ������ȡ���ᣬ���ᣨH3BO3����һ��Ƭ��״�ṹ��ɫ���壬���ڵġ�H3BO3����֮��ͨ�������������ͼ2�����������й�˵���в���ȷ����
A�����ᾧ�����ڷ��Ӿ���
B��H3BO3����������������������ӽ��ȶ�
C�������и�ԭ��������Ϊ8�����ȶ��ṹ
D.1mol H3BO3�������3mol�����
��������1��������Ӧ���к���4�ֲ�ͬ�������ӵ�Ԫ����NaԪ�أ����ݹ���ԭ����д���̬ԭ�Ӻ�������Ų�ʽ��
��2��ͬһ����Ԫ���У�Ԫ�صĵ�һ����������ԭ����������������������ƣ����ڢ�A�塢�ڢ�A��Ԫ�صĵ�һ�����ܴ�������Ԫ�أ�
��3���ٸ��ݼ۲���ӶԻ�������ȷ���ӻ���ʽ��
��B��Nԭ��֮��Ħм����ӱ��縺�Խϴ��ԭ��������
�۵������۵�ϸߣ������ͻ���ϣ��ʴ�Ϊ���ͻ���ϣ�
��4�����йµ��ӶԵ�ԭ�Ӻͺ��пչ����ԭ��֮�������λ����
��5��A�����ݹ������жϾ������ͣ�
B�����ӵ��ȶ����뻯ѧ���йأ�
C���û�������Hԭ�Ӳ��ܴﵽ8���ӽṹ��
D�����þ�̯�����㺬1molH3BO3�ľ����е������
��2��ͬһ����Ԫ���У�Ԫ�صĵ�һ����������ԭ����������������������ƣ����ڢ�A�塢�ڢ�A��Ԫ�صĵ�һ�����ܴ�������Ԫ�أ�
��3���ٸ��ݼ۲���ӶԻ�������ȷ���ӻ���ʽ��
��B��Nԭ��֮��Ħм����ӱ��縺�Խϴ��ԭ��������
�۵������۵�ϸߣ������ͻ���ϣ��ʴ�Ϊ���ͻ���ϣ�
��4�����йµ��ӶԵ�ԭ�Ӻͺ��пչ����ԭ��֮�������λ����
��5��A�����ݹ������жϾ������ͣ�
B�����ӵ��ȶ����뻯ѧ���йأ�
C���û�������Hԭ�Ӳ��ܴﵽ8���ӽṹ��
D�����þ�̯�����㺬1molH3BO3�ľ����е������
����⣺��1��������Ӧ���к���4�ֲ�ͬ�������ӵ�Ԫ����NaԪ�أ�NaԪ�غ�����11�����ӣ����ݹ���ԭ��֪�����̬ԭ�Ӻ�������Ų�ʽΪ��1s22s22p63s1��
�ʴ�Ϊ��1s22s22p63s1��
��2��ͬһ����Ԫ���У�Ԫ�صĵ�һ����������ԭ����������������������ƣ����ڢ�A�塢�ڢ�A��Ԫ�صĵ�һ�����ܴ�������Ԫ�أ������⼸��Ԫ�ص�һ�����ܴ�С˳���ǣ�N��O��C��B��
�ʴ�Ϊ��N��O��C��B��
��3���ٸýṹ֪��ÿ��Nԭ�Ӻ�Bԭ�Ӷ�����3�����۵��������Բ���sp2�ӻ���
�ʴ�Ϊ��sp2��sp2��
��B��Nԭ��֮��Ħм����ӱ��縺�Խϴ��ԭ��������NԪ�صĵ縺�Դ���BԪ�أ����Ա�Nԭ��ǿ��������
�ʴ�Ϊ��N��
��ԭ�Ӿ����۵�ϸߣ������ͻ���ϣ�
�ʴ�Ϊ���ͻ���ϣ�
��4�����йµ��ӶԵ�ԭ�Ӻͺ��пչ����ԭ��֮�������λ����1mol��������к���6NA����λ�����γ���λ��ʱ���ṩ�¶Ե��ӵ�ԭ����Oԭ�ӣ�
�ʴ�Ϊ��Oԭ�ӣ�
��5��A�������ᾧ���д���H3BO3���ӣ��������ڷ��Ӿ��壬��A��ȷ��
B������Ƿ��Ӽ����ã�����ӵ��ȶ����أ���B����
C���÷�������ԭ�Ӳ����γ�8�����ȶ��ṹ����C����
D.1����������γ���6���������ÿ�������2��������ӹ��õģ�����ƽ����3�����������1molH3BO3�ľ�������3mol�������D��ȷ��
��ѡ��BC��
�ʴ�Ϊ��1s22s22p63s1��
��2��ͬһ����Ԫ���У�Ԫ�صĵ�һ����������ԭ����������������������ƣ����ڢ�A�塢�ڢ�A��Ԫ�صĵ�һ�����ܴ�������Ԫ�أ������⼸��Ԫ�ص�һ�����ܴ�С˳���ǣ�N��O��C��B��
�ʴ�Ϊ��N��O��C��B��
��3���ٸýṹ֪��ÿ��Nԭ�Ӻ�Bԭ�Ӷ�����3�����۵��������Բ���sp2�ӻ���
�ʴ�Ϊ��sp2��sp2��
��B��Nԭ��֮��Ħм����ӱ��縺�Խϴ��ԭ��������NԪ�صĵ縺�Դ���BԪ�أ����Ա�Nԭ��ǿ��������
�ʴ�Ϊ��N��
��ԭ�Ӿ����۵�ϸߣ������ͻ���ϣ�
�ʴ�Ϊ���ͻ���ϣ�
��4�����йµ��ӶԵ�ԭ�Ӻͺ��пչ����ԭ��֮�������λ����1mol��������к���6NA����λ�����γ���λ��ʱ���ṩ�¶Ե��ӵ�ԭ����Oԭ�ӣ�
�ʴ�Ϊ��Oԭ�ӣ�
��5��A�������ᾧ���д���H3BO3���ӣ��������ڷ��Ӿ��壬��A��ȷ��
B������Ƿ��Ӽ����ã�����ӵ��ȶ����أ���B����
C���÷�������ԭ�Ӳ����γ�8�����ȶ��ṹ����C����
D.1����������γ���6���������ÿ�������2��������ӹ��õģ�����ƽ����3�����������1molH3BO3�ľ�������3mol�������D��ȷ��
��ѡ��BC��
���������⿼���������Ų����ɡ��ӻ�������ۡ�������һ�����ܴ�С���жϵȣ��Ѷ��еȣ�ע����������ڻ�ѧ������һ�����ܹ����е��쳣����Ϊ�״��㣮

��ϰ��ϵ�д�
 �㽭��У��ʦ���ϵ�д�
�㽭��У��ʦ���ϵ�д�
�����Ŀ
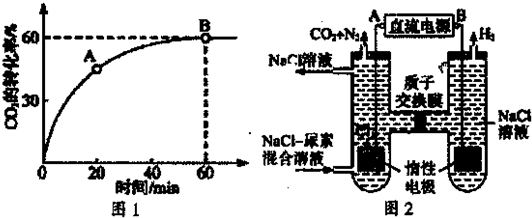

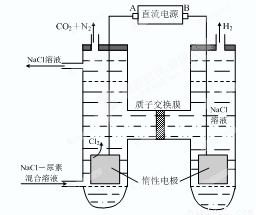
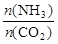 ��4ʱ��CO2��ת������ʱ��ı仯��ϵ����ͼ��ʾ��
��4ʱ��CO2��ת������ʱ��ı仯��ϵ����ͼ��ʾ��