��Ŀ����
��ͼΪijЩ��������֮���ת����ϵ����֪��A��B��I�к�����ͬ���������Ҷ���XY2�ͻ������I��ʵ���ҳ��õĸ������CΪֱ���ͷ��ӣ�E��FΪ�ǽ������嵥�ʡ��밴Ҫ����գ�
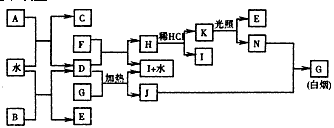
��1����B�ĵ���ʽ��___________����K�Ľṹʽ��_______________��
��2��D��G��Ӧ�Ļ�ѧ����ʽ��____________________________________��
��3����֪C��ȼ������1300kJ/mol����ʾC��ȼ���ȵ��Ȼ�ѧ����ʽ��__________________________��
��4����G����ˮ�����Һ�������������ҺG�����������ӵIJ���������_______________________��
��5��������0.1mol/L��J��Һ��c(H+)/c(OH-)=1��10-8�����������������_________________��
A������Һ��pH=11
B������Һ�е����ʵ������������Ũ��0.1mol/L
C������Һ��ˮ�������c(H+)��c(OH-)�˻�Ϊ1��10-22
D��pH=3��������ҺV1 L���0.1mol/L��J��ҺV2 L���,�������ҺpH=7����V1>V2
E����������Һ��ˮϡ��100����pHֵΪ9
��6������F�ڹ�ҵ������Ҫ����;��___________________�����������֣�
��2��D��G��Ӧ�Ļ�ѧ����ʽ��____________________________________��
��3����֪C��ȼ������1300kJ/mol����ʾC��ȼ���ȵ��Ȼ�ѧ����ʽ��__________________________��
��4����G����ˮ�����Һ�������������ҺG�����������ӵIJ���������_______________________��
��5��������0.1mol/L��J��Һ��c(H+)/c(OH-)=1��10-8�����������������_________________��
A������Һ��pH=11
B������Һ�е����ʵ������������Ũ��0.1mol/L
C������Һ��ˮ�������c(H+)��c(OH-)�˻�Ϊ1��10-22
D��pH=3��������ҺV1 L���0.1mol/L��J��ҺV2 L���,�������ҺpH=7����V1>V2
E����������Һ��ˮϡ��100����pHֵΪ9
��6������F�ڹ�ҵ������Ҫ����;��___________________�����������֣�
(1)��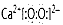 ��H-O-Cl
��H-O-Cl
(2)2NH4Cl+Ca(OH)2 CaCl2+2NH3��+2H2O
CaCl2+2NH3��+2H2O
(3)C2H2��g��+5/2 O2��g��= 2CO2��g��+H2O��l����H=-1300kJ/mol
(4)ȡ������G��Һ���Թ��У���������������������Һ�������Թܣ���ʪ��ĺ�ɫʯ����ֽ�����Թܿڣ�����ֽ�����ɫ������NH4+
(5)BE
(6)��Ư�ۣ������ᡢ����Ư��Һ��
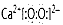 ��H-O-Cl
��H-O-Cl(2)2NH4Cl+Ca(OH)2
 CaCl2+2NH3��+2H2O
CaCl2+2NH3��+2H2O (3)C2H2��g��+5/2 O2��g��= 2CO2��g��+H2O��l����H=-1300kJ/mol
(4)ȡ������G��Һ���Թ��У���������������������Һ�������Թܣ���ʪ��ĺ�ɫʯ����ֽ�����Թܿڣ�����ֽ�����ɫ������NH4+
(5)BE
(6)��Ư�ۣ������ᡢ����Ư��Һ��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ







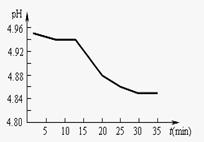 ��2��SO2���ŷ�������������Ҫ���ء�ij��������
��2��SO2���ŷ�������������Ҫ���ء�ij��������