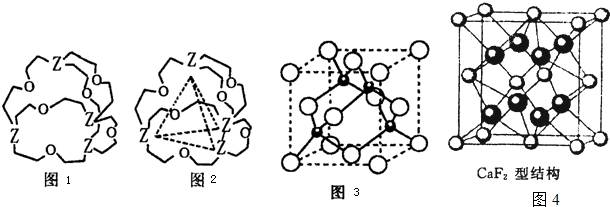
��Ŀ����
[��ѧ--ѡ�����ʽṹ������]��֪A��B��C��D��E����Ԫ�����ڱ��е�ǰ20��Ԫ�أ����ǵ�ԭ��������������B��C��Dͬ���ڣ�A��Dͬ���壬B��C��D������������ˮ����������Ͼ��ܷ�����Ӧ�����κ�ˮ��EԪ�ص�ԭ�Ӻ����20�ֲ�ͬ�˶�״̬�ĵ��ӣ���E��ԭ��������D��4��
��1��B��C�ĵ�һ�����ܽϴ���� ����Ԫ�ط��ţ���
��2��A���⻯��ķ��ӿռ乹��Ϊ ��������ԭ�Ӳ�ȡ �ӻ���
��3��A��D���⻯���У��е�ϸߵ��� ���ѧʽ������ԭ���� ��
��4������A��BԪ����ɣ��Һ��зǼ��Լ��Ļ������� ���ѧʽ����
��5��E�ĺ�������Ų�ʽ�� ��
��6��B������������Ӧ��ˮ�������Һ��C���ʷ�Ӧ�Ļ�ѧ����ʽ�� ��
��7��E������A������ȼ��ʱ�õ�һ�ְ�ɫ���壬�侧��ľ����ṹ����ͼ��ʾ����þ���Ļ�ѧʽΪ ��

���𰸡�������A��B��C��D��E����Ԫ�����ڱ��е�ǰ20��Ԫ�أ����ǵ�ԭ��������������B��C��Dͬ���ڣ�B��C��D������������ˮ����������Ͼ��ܷ�����Ӧ�����κ�ˮ��
��BΪNa��CΪAl��EԪ�ص�ԭ�Ӻ����20�ֲ�ͬ�˶�״̬�ĵ��ӣ���EΪCa����E��ԭ��������D��4����DΪS��A��Dͬ���壬��AΪO��Ȼ������Ԫ�ؼ��䵥�ʡ�����������ʡ��ṹ�������
����⣺A��B��C��D��E����Ԫ�����ڱ��е�ǰ20��Ԫ�أ����ǵ�ԭ��������������B��C��Dͬ���ڣ�B��C��D������������ˮ����������Ͼ��ܷ�����Ӧ�����κ�ˮ��
��BΪNa��CΪAl��EԪ�ص�ԭ�Ӻ����20�ֲ�ͬ�˶�״̬�ĵ��ӣ���EΪCa����E��ԭ��������D��4����DΪS��A��Dͬ���壬��AΪO��
��AΪO��BΪNa��CΪAl��DΪS��EΪCa��
��1��BΪNa��CΪAl��Al���������Ӱ�����Ϊ�ȶ��ṹ����Al�ĵ�һ�����ܽϴʴ�Ϊ��Al��
��2��A���⻯��ΪH2O�����ӹ���ΪV�ͻ������ͣ�O��2�Թ¶Ե��Ӻ��������۵��������ӻ�����Ϊsp3��
�ʴ�Ϊ��V�ͻ������ͣ�sp3��
��3��H2O��H2S��Ƚϣ�ˮ����֮������γ��������H2S����֮�䲻���γ��������ˮ�ķе�ߣ�
�ʴ�Ϊ��H2O��ˮ����֮������γ��������H2S����֮�䲻���γ������
��4������A��BԪ����ɵĻ�������Na2O��Na2O2���Һ��зǼ��Լ��Ļ�����ΪNa2O2���ʴ�Ϊ��Na2O2��
��5��EΪCa�������Ų�ʽΪ1S22S22P63S23P64S2���ʴ�Ϊ��1S22S22P63S23P64S2��
��6��B������������Ӧ��ˮ����ΪNaOH����Al��Ӧ�Ļ�ѧ����ʽΪ2Al+2NaOH+6H2O=2Na[Al��OH��4]+3H2��
��2Al+2NaOH+2H2O=2NaAlO2+3H2����
�ʴ�Ϊ��2Al+2NaOH+6H2O=2Na[Al��OH��4]+3H2����2Al+2NaOH+2H2O=2NaAlO2+3H2����
��7���ɾ���ľ����ṹͼ��֪��O22-ռ���������ģ�����Ϊ12× +1=4��������ռ�ݶ�������ģ�����Ϊ8×
+1=4��������ռ�ݶ�������ģ�����Ϊ8× +6×
+6× =4��������Ϊ1��1��
=4��������Ϊ1��1��
��ѧʽΪCaO2���ʴ�Ϊ��CaO2��
���������⿼��λ�á��ṹ�����ʵĹ�ϵ��Ԫ�ص��ƶ��ǽ����Ĺؼ�������Ϥ���ʽṹ�����ʵĹ�ϵ����ɣ��ѶȽϴ�
��BΪNa��CΪAl��EԪ�ص�ԭ�Ӻ����20�ֲ�ͬ�˶�״̬�ĵ��ӣ���EΪCa����E��ԭ��������D��4����DΪS��A��Dͬ���壬��AΪO��Ȼ������Ԫ�ؼ��䵥�ʡ�����������ʡ��ṹ�������
����⣺A��B��C��D��E����Ԫ�����ڱ��е�ǰ20��Ԫ�أ����ǵ�ԭ��������������B��C��Dͬ���ڣ�B��C��D������������ˮ����������Ͼ��ܷ�����Ӧ�����κ�ˮ��
��BΪNa��CΪAl��EԪ�ص�ԭ�Ӻ����20�ֲ�ͬ�˶�״̬�ĵ��ӣ���EΪCa����E��ԭ��������D��4����DΪS��A��Dͬ���壬��AΪO��
��AΪO��BΪNa��CΪAl��DΪS��EΪCa��
��1��BΪNa��CΪAl��Al���������Ӱ�����Ϊ�ȶ��ṹ����Al�ĵ�һ�����ܽϴʴ�Ϊ��Al��
��2��A���⻯��ΪH2O�����ӹ���ΪV�ͻ������ͣ�O��2�Թ¶Ե��Ӻ��������۵��������ӻ�����Ϊsp3��
�ʴ�Ϊ��V�ͻ������ͣ�sp3��
��3��H2O��H2S��Ƚϣ�ˮ����֮������γ��������H2S����֮�䲻���γ��������ˮ�ķе�ߣ�
�ʴ�Ϊ��H2O��ˮ����֮������γ��������H2S����֮�䲻���γ������
��4������A��BԪ����ɵĻ�������Na2O��Na2O2���Һ��зǼ��Լ��Ļ�����ΪNa2O2���ʴ�Ϊ��Na2O2��
��5��EΪCa�������Ų�ʽΪ1S22S22P63S23P64S2���ʴ�Ϊ��1S22S22P63S23P64S2��
��6��B������������Ӧ��ˮ����ΪNaOH����Al��Ӧ�Ļ�ѧ����ʽΪ2Al+2NaOH+6H2O=2Na[Al��OH��4]+3H2��
��2Al+2NaOH+2H2O=2NaAlO2+3H2����
�ʴ�Ϊ��2Al+2NaOH+6H2O=2Na[Al��OH��4]+3H2����2Al+2NaOH+2H2O=2NaAlO2+3H2����
��7���ɾ���ľ����ṹͼ��֪��O22-ռ���������ģ�����Ϊ12×
 +1=4��������ռ�ݶ�������ģ�����Ϊ8×
+1=4��������ռ�ݶ�������ģ�����Ϊ8× +6×
+6× =4��������Ϊ1��1��
=4��������Ϊ1��1����ѧʽΪCaO2���ʴ�Ϊ��CaO2��
���������⿼��λ�á��ṹ�����ʵĹ�ϵ��Ԫ�ص��ƶ��ǽ����Ĺؼ�������Ϥ���ʽṹ�����ʵĹ�ϵ����ɣ��ѶȽϴ�

��ϰ��ϵ�д�
 �Ͻ�ƽ���Ȿϵ�д�
�Ͻ�ƽ���Ȿϵ�д� ����ѧ��Ӧ�����ϵ�д�
����ѧ��Ӧ�����ϵ�д�
�����Ŀ