��Ŀ����
����Ŀ���������庬���϶��Ԫ��֮һ���Ļ�������ҩ��������ũҩ����ȷ�����;�dz��㷺���ش��������⣺
��1����̬��ԭ�ӵĺ�������Ų�ʽΪ____________________��
��2��P4S3������������,����ӽṹ��ͼ����ʾ��
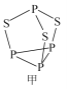

����һ����������_____________��;�縺������_____________��(�>����<��)��
��P4S3��������ԭ�ӵ��ӻ��������Ϊ_____________��
��ÿ��P4S3�����к��µ��ӶԵ���ĿΪ______________��
��3��N��P��As��Sb���ǵ�VA���Ԫ�ء�
������Ԫ�ص��⻯��ķе��ϵ��ͼ����ʾ���е㣺PH3<NH3,��ԭ����____________________;�е���PH3<AsH3<SbH3����ԭ����_____________________________________________________��
��ij�ִ��Ե������ľ����ṹ��ͼ����ʾ,�û�����Ļ�ѧʽΪ_________________��
��4�������۵�Ϊ2000�������뾧��軥Ϊ�ȵ����������������ṹ��ͼ����ʾ��
��ͼ��A���B���ԭ�����������ͼ����ʾ����C���ԭ���������Ϊ_______________��
������������ܶ�Ϊ��g��cm-3��NA��ʾ�����ӵ���������ֵ����þ����о��������������ԭ��֮��ľ���Ϊ_____________________________cm��
���𰸡� 1s22s22p63s23p3��[Ne]3s23p3 > < sp3 10 NH3���Ӽ���ڷ��Ӽ���� ��Է����������������Ӽ�������������ǿ Fe3N (![]() ��
��![]() ��
��![]() )
) ![]()
��������������������⿼�����ʽṹ�����ʣ��漰ԭ�Ӻ�������Ų�ʽ����д����һ�����ܺ͵縺�ԵıȽϣ�ԭ���ӻ���ʽ���жϣ����ʷе�ߵ͵ıȽϣ����廯ѧʽ��ȷ���Լ������ļ��㡣
��1��P�ĺ˵����Ϊ15��Pԭ�Ӻ��������Ϊ15�����ݹ���ԭ������̬Pԭ�ӵĺ�������Ų�ʽΪ1s22s22p63s23p3��[Ne] 3s23p3��
��2����Pԭ�ӵļ۵����Ų�ʽΪ3s23p3��Sԭ�ӵļ۵����Ų�ʽΪ3s23p4��Pԭ�ӵ�3p���ڰ�������ȶ�����һ�����ܣ�P![]() S���ǽ�������P
S���ǽ�������P![]() S���縺�ԣ�P
S���縺�ԣ�P![]() S��
S��
���ɽṹ֪��P4S3��ÿ��Sԭ���γ�2���Ҽ���Sԭ���ϻ���2�Թµ��Ӷԣ���Sԭ��Ϊsp3�ӻ���
���ɽṹ֪��ÿ��Pԭ���γ�3�Թ��õ��Ӷԣ�ÿ��Pԭ������1�Թµ��Ӷԣ�ÿ��Sԭ���γ�2�Թ��õ��Ӷԣ�ÿ��Sԭ������2�Թµ��Ӷ���ÿ��P4S3�к��еŵ��Ӷ���Ϊ4![]() 1+3
1+3![]() 2=10��
2=10��
��3�����е���PH3![]() NH3��ԭ���ǣ�NH3���Ӽ���������PH3���Ӽ䲻����������е���PH3<AsH3<SbH3��ԭ���ǣ�PH3��AsH3��SbH3�����ڷ��Ӿ��壬��Է����������������Ӽ�������������ǿ���е����ߡ�
NH3��ԭ���ǣ�NH3���Ӽ���������PH3���Ӽ䲻����������е���PH3<AsH3<SbH3��ԭ���ǣ�PH3��AsH3��SbH3�����ڷ��Ӿ��壬��Է����������������Ӽ�������������ǿ���е����ߡ�
��������̯������Fe��12![]() +2
+2![]() +3=6��Nȫ�ھ����ڲ���N��2��N��Fe����N��N��=6:2=3:1����ѧʽΪFe3N��
+3=6��Nȫ�ھ����ڲ���N��2��N��Fe����N��N��=6:2=3:1����ѧʽΪFe3N��
��4�������վ���ͼʾ������ϵ�Լ�A��B�����꣬ѡA��Ϊ���յ㣬�۲�C���ھ�����λ�ã���Խ���![]() ��������A��B�����������֪C������Ϊ��
��������A��B�����������֪C������Ϊ��![]() ��
�� ![]() ��
�� ![]() ����
����
��������̯������1��������Al��8![]() +6
+6![]() =4��P��4������Ļ�ѧʽΪAlP��1mol���������Ϊ58g���辧���ı߳�Ϊx���������Ϊx3�����g/cm3
=4��P��4������Ļ�ѧʽΪAlP��1mol���������Ϊ58g���辧���ı߳�Ϊx���������Ϊx3�����g/cm3![]() NA=58g�����x=
NA=58g�����x=![]() cm�������о��������������ԭ��֮��ľ���Ϊ
cm�������о��������������ԭ��֮��ľ���Ϊ![]() x=
x=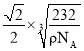 cm��
cm��

 �ǻۿ����ܾ�100�ֵ�Ԫ���ؼ��ϵ�д�
�ǻۿ����ܾ�100�ֵ�Ԫ���ؼ��ϵ�д� ��Ԫ������ĩ��ϵ�д�
��Ԫ������ĩ��ϵ�д�����Ŀ��ij��ѧ�о���ѧϰС�����ԭ����γ������������ʵ�鷽������������̽����
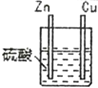
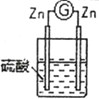
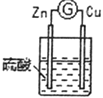
(1)����д�й�ʵ�����ó���ؽ��ۡ�
��� | ʵ��װ�� | ʵ������ |
1 |
| п�����ܽ⣬�������������ɣ�ͭ������������ |
2 |
| ��п�����ܽ⣬��������������ɣ�������ָ�벻ƫת |
3 |
| ͭ�������������______________________��������ָ��___________________ |
��ͨ��ʵ��2��3���ɵó�ԭ��ص��γ�������______________________________��
��ͨ��ʵ��1��3���ɵó�ԭ��ص��γ�������______________________________��
������3װ�������ỻ���Ҵ���������ָ�뽫������ƫת���Ӷ��ɵó�ԭ����γ�������___________________��
(2)�ֱ�д��ʵ��3��Zn����Cu���Ϸ����ĵ缫��Ӧʽ��
Zn����______________________________��
Cu����______________________________��
(3)ʵ��3�ĵ����Ǵ�________������(�Zn����Cu��)����Ӧ����������0.4mol���ӷ�����ת�ƣ���Zn�缫��������___________g��
����Ŀ������ԭCO2�ǽ������ЧӦ����Դ�������Ҫ�ֶ�֮һ���о���������Cu/ZnO���������£���CO2��ͨ��H2�����߿ɷ�����������ƽ�з�Ӧ��
��Ӧ�� CO2(g)+3H2(g)![]() CH3OH(g)+H2O(g) ��H1=-53.7 kJ��mol-1��
CH3OH(g)+H2O(g) ��H1=-53.7 kJ��mol-1��
��Ӧ�� CO2(g)+H2(g)![]() CO(g)+H2O(g)�� ����H2=+41.2 kJ��mol-1
CO(g)+H2O(g)�� ����H2=+41.2 kJ��mol-1
ijʵ���ҿ���һ����CO2��H2��ʼͶ�ϱ�������ͬѹǿ��,������ͬ��Ӧʱ��������ʵ�����ݣ����С��״�ѡ���ԡ���ָת����CO2�����ɼ״��İٷֱ�����
��Ӧ��� | T/K | ���� | CO2ת����/% | �״�ѡ����/% |
�� | 543 | Cu/ZnO���װ� | 12.3 | 42.3 |
�� | 543 | Cu/ZnO����Ƭ | 10.9 | 72.7 |
�� | 553 | Cu/ZnO���װ� | 15.3 | 39.1 |
�� | 553 | Cu/ZnO����Ƭ | 12.0 | 71.6 |
��1��CO2�ĵ���ʽ��_____________��
��2����Ӧ����ƽ�ⳣ������ʽ��K=______��
��3���ԱȢٺ͢ۿɷ��֣�ͬ�����������£��¶����ߣ�CO2ת�������ߣ� ���״���ѡ����ȴ���ͣ�����ͼ״�ѡ���Խ��͵Ŀ���ԭ��_______________��
�ԱȢ١��ڿɷ��֣���ͬ���¶��£�����Cu/ZnO����ƬʹCO2ת���ʽ����� ���״���ѡ����ȴ��ߣ�����ͼ״���ѡ������ߵĿ���ԭ��____________��
��4�����������CO2ת��ΪCH3OHƽ��ת���ʵĴ�ʩ��____��
a��ʹ��Cu/ZnO���װ�������
b��ʹ��Cu/ZnO����Ƭ������
c���ͷ�Ӧ�¶�
d��Ͷ�ϱȲ���,���ӷ�Ӧ���Ũ��
e������CO2��H2�ij�ʼͶ�ϱ�