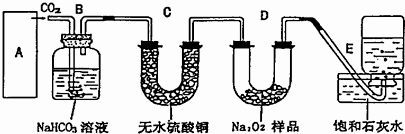
��Ŀ����
�����ͼ��ʾ��ʵ��װ�ã�����̨�ȸ���������ȥδ����

�ش��������⣺
��1���ڼ��ȹ������ܹ۲쵽������ɫ�������ձ���______����������ձ������ձ��з�����Ӧ�����ӷ���ʽ�ǣ�______��ͨ��ʵ��ɱȽϳ�Na2CO3��NaHCO3���ֹ��壬______���ȶ���
��2����ijNa2CO3�����л�������NaHCO3���ʣ���ȥ���ʵķ���Ϊ______����ijNaHCO3��Һ�л�������Na2CO3���ʣ���ȥ���ʵĻ�ѧ����ʽΪ______��

�ش��������⣺
��1���ڼ��ȹ������ܹ۲쵽������ɫ�������ձ���______����������ձ������ձ��з�����Ӧ�����ӷ���ʽ�ǣ�______��ͨ��ʵ��ɱȽϳ�Na2CO3��NaHCO3���ֹ��壬______���ȶ���
��2����ijNa2CO3�����л�������NaHCO3���ʣ���ȥ���ʵķ���Ϊ______����ijNaHCO3��Һ�л�������Na2CO3���ʣ���ȥ���ʵĻ�ѧ����ʽΪ______��
��1���ڼ��ȹ����У�̼���������ȷֽ����ɶ�����̼��������̼�����ʯ��ˮ����ǣ������ձ����г��ְ�ɫ��������Ӧ�����ӷ���ʽΪ��Ca2++CO2+2OH-=CaCO3��+H2O��ͨ����ʵ��֤����̼�����ȶ��Դ���̼�����ƣ�
�ʴ�Ϊ����Ca2++CO2+2OH-=CaCO3��+H2O��Na2CO3��
��2����ijNa2CO3�����л�������NaHCO3���ʣ�����ͨ�����ȷ���ʹ̼�����Ʒֽ�����̼���ƶ���ȥ����ijNaHCO3��Һ�л�������Na2CO3���ʣ�����ͨ�������̼���壬ʹ̼�����������̼��Ӧ����̼�����ƣ���Ӧ�Ļ�ѧ����ʽΪ��Na2CO3+CO2+H2O=2NaHCO3��
�ʴ�Ϊ�����ȣ�Na2CO3+CO2+H2O=2NaHCO3��
�ʴ�Ϊ����Ca2++CO2+2OH-=CaCO3��+H2O��Na2CO3��
��2����ijNa2CO3�����л�������NaHCO3���ʣ�����ͨ�����ȷ���ʹ̼�����Ʒֽ�����̼���ƶ���ȥ����ijNaHCO3��Һ�л�������Na2CO3���ʣ�����ͨ�������̼���壬ʹ̼�����������̼��Ӧ����̼�����ƣ���Ӧ�Ļ�ѧ����ʽΪ��Na2CO3+CO2+H2O=2NaHCO3��
�ʴ�Ϊ�����ȣ�Na2CO3+CO2+H2O=2NaHCO3��

��ϰ��ϵ�д�
 ����С��ʿ���������ϵ�д�
����С��ʿ���������ϵ�д�
�����Ŀ