��Ŀ����
����Ŀ��������ҩ��Υ���涨�����빤ҵ�á����ʴ�������ҽ�ñ�����(C2H8O2)�����ϣ����ڡ���������ע��Һ�������������¶�������������˥Ъ�����������ʴ��ֳ��Ҷ����ѣ�����ʽΪC4H10O3(HO-CH2-CH2-O-CH2-CH2-OH)�����ʴ���һ����Ҫ�Ļ���ԭ�ϣ���;ʮ�ֹ㷺�����ʴ�һ��ĺϳ�·��Ϊ��

��ش��������⣺
��1�����й��ڡ��������͡����ʴ��������Ҷ��������й�˵����ȷ����____________
A.���������Ҷ�����ͬϵ��
B.���Ǿ�����ͬ�������Ŀ�Ĺ����ţ���������ȫ��ͬ
C.�����������͡����ʴ����ڲ������ڶ��ܱ�����Ϊ����
D.���롰���������͡����ʴ����ɲ��÷�Һ�ķ���
��2������I��ʯ�ͼӹ��г��ò��裬������Ϊ_______��
��3��������B������C�Ĺ�������������Ʋ��û���������E��E�����ڽ������ид��ʵ�����Ʊ�E�Ļ�ѧ����ʽ___________����Ϊ���ܵõ����ʴ�D������B������C�ķ�Ӧ������_________���÷�Ӧ����________(�Ӧ����)��д��B��������E�Ļ�ѧ����ʽ______��
��4����Ӧ��Ļ�ѧ����ʽΪ��____________��
��5��A��һ��ͬϵ��ṹ��ʽΪ ����ϵͳ��������������_______________��
����ϵͳ��������������_______________��
���𰸡� A �ѽ� CaC2+2H2O=Ca(OH)2+CH��CH NaOHˮ��Һ ˮ���ȡ�� CH2BrCH2Br+2NaOH![]() CH��CH+2NaBr+2H2O 2HO-CH2-CH2-OH
CH��CH+2NaBr+2H2O 2HO-CH2-CH2-OH![]() HO-CH2-CH2-O-CH2-CH2-OH+H2O 2,3-���һ�-1-��ϡ
HO-CH2-CH2-O-CH2-CH2-OH+H2O 2,3-���һ�-1-��ϡ
��������(1)A�����������Ҷ���������ͬ�Ĺ�����-OH������Ŀ��ͬ������Ϊ���������ͨʽ��ͬ����A��ȷ��B�����ʴ�������2��-OH���������Ѽ�������������Ҷ������еĹ����Ų�ͬ����B����C������������������Ϊ���ᣬ���ʴ�������HOOCHCH2-O-CH2CHO0H����C����D������������ʴ����ܣ��������÷�Һ�������룬��D����ѡA��
(2)��ʯ���ѽ�������ϩ��
(3)BrCH2CH2Br����������ˮ��Һ�������·���ȡ����Ӧ����HOCH2CH2OH������������Ʋ��ûᷢ����ȥ��Ӧ������Ȳ����Ӧ����ʽΪ��CH2BrCH2Br+2NaOH![]() HC��CH��+2NaBr��
HC��CH��+2NaBr��
(4)��Ӧ�����Ҷ�����Ũ���ᡢ���������·������Ӽ���ˮ����HOCH2CH2-O-CH2CH2OH����Ӧ����ʽΪ��2HO-CH2-CH2-OH![]() 2HO-CH2-CH2-O-CH2-CH2-OH+H2O��
2HO-CH2-CH2-O-CH2-CH2-OH+H2O��
(5)����ϵͳ�������л���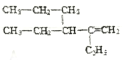 �Ļ�ѧ����Ϊ2,3-���һ�-1-��ϡ��
�Ļ�ѧ����Ϊ2,3-���һ�-1-��ϡ��

����Ŀ��ʵ���ҳ���ʯ��ʯ��ϡ������ȡ������̼��
̽��һ ���巢��װ�õ�ѡ��

��1��д��ͼ�д�������������ƣ�a_____________��b_______________��
��2��ʵ������ȡ������̼���壬�������ռ�װ�÷ֱ�ѡ��______ ��______ ������ĸ�������鼯���ķ�����___________________________________��
̽���� ҩƷ��ѡ��
С��������ҩƷ�������о���ʵ���¼���£�
��� | ҩ Ʒ | ʵ������ |
�� | ��״ʯ��ʯ��ϡ���� | ���������������� |
�� | ��״ʯ��ʯ��ϡ���� | �����������ʻ�������ֹͣ |
�� | ��ĩ״ʯ��ʯ��ϡ���� | �����������ʺܿ� |
��3������ʵ��٢ۣ���̽��_____________________�Բ���������̼���ʵ�Ӱ�죻
��4������ʵ��______����̽����ͬ����Բ���������̼���ʵ�Ӱ�죻
��5��С��ѡ��ڢ���ҩƷ����ȡ������̼�����鷴Ӧ�Ļ�ѧ����ʽΪ___________��
̽���� ���ɶ�����̼���IJⶨ
ʵ���ҳ�ͨ����������;���������ɶ�����̼����
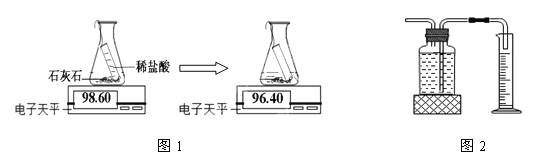
��6��;��һ����ͼ1������ͼ��֪����������̼������Ϊ________g��
;��������ͼ2����ͨ����ˮ��������ɶ�����̼�������
��7����������;���Ƚϣ�����Ϊ����;��ʵ������Ϊȷ��������___________________��
