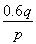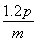��Ŀ����
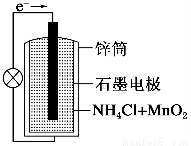
���Ե�ؾ��������ŵ��������ص㣬����õ��㷺Ӧ�á�п���̼��Ե��������������ҺΪ���Һ������ܷ�ӦʽΪ��
Zn��s����2MnO2��s����H2O��l��=Zn��OH��2��s����Mn2O3��s��
����˵��������ǣ� ��
A����ع���ʱ��пʧȥ����
B����������ĵ缫��ӦʽΪ2MnO2��s����H2O��l����2e��=
Mn2O3��s����2OH����aq��
C����ع���ʱ������������ͨ�����·����
D�����·��ÿͨ��0.2 mol���ӣ�п�����������ϼ���6.5 g
C
�������������ܷ�Ӧʽ��Ԫ�ػ��ϼ۵ı仯����֪��ع���ʱ��пʧ��������Դ�ĸ�������������MnO2�����õ��ӵĻ�ԭ��Ӧ����˵�ع���ʱ�����·�ϵ����ɵ�صĸ����������������A��B������ȷ��C������ɹ�ϵʽZn��2e����֪��Dѡ����ȷ��

��֪ij����̬��ʯȼ�Ϻ���̼��������Ԫ�ء�Ϊ��
�ⶨ����ȼ����̼��������Ԫ�ص������������ɽ���̬ȼ�Ϸ���������������ȼ�գ���ʹ����������ȫ��ͨ����ͼ��ʾ��װ�ã��õ�������е�ʵ�����ݣ�U�ι��и����ֻ����ˮ�����Ҽ��������������ȫ�����գ���
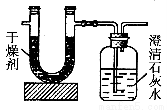
| ʵ��ǰ | ʵ��� |
���������U�ιܣ������� | 101.1g | 102��9g |
��ʯ��ˮ�����ƿ�������� | 312.0g | 314.2g |
����ʵ��������
��1��ʵ����Ϻ���������ˮ������Ϊ________g��������ƿ������һ�����Σ�������Ϊ________g��
��2�����ɵ�ˮ����Ԫ�ص�����Ϊ________g��
��3�����ɵ�CO2��̼Ԫ�ص�����Ϊ________g��
��4����̬��ʯȼ����̼Ԫ������Ԫ�ص�������Ϊ________��