��Ŀ����
��8�֣�̼�ǻ�������������Ԫ�أ��䵥�ʼ������������������������Ҫ��Դ���ʡ�
��1��ʹCl2��H2O(g)ͨ�����ȵ�̿�㣬����HCl��CO2������1molCl2���뷴Ӧʱ�ͷų�145kJ������д���÷�Ӧ���Ȼ�ѧ����ʽ�� ��
��2����ѧ������̫���ֽܷ�ˮ���ɵ������ڴ����������������̼��Ӧ���ɼ״�����֪H2(g)��CO(g)��CH3OH(l)��ȼ���Ȧ�H�ֱ�Ϊ��285.8kJ��mol-1����283.0kJ��mol-1�ͣ�726.5kJ��mol-1����
����̫���ֽܷ�10molˮ���ĵ�������_____________kJ��
�ڼ״�����ȫȼ������һ����̼��Һ̬ˮ���Ȼ�ѧ����ʽΪ_______ ____ ��
��3����֪��Fe2O3(s)��3C(ʯī)��2Fe(s)��3CO(g) ��H����489.0 kJ��mol-1
CO(g)��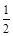 O2(g) ��CO2(g)����H����283.0 kJ��mol-1
O2(g) ��CO2(g)����H����283.0 kJ��mol-1
C(ʯī)��O2(g) ��CO2(g)����H����393.5 kJ��mol-1
��4Fe(s)��3O2(g) ��2Fe2O3(s)����H�� ��
��1��ʹCl2��H2O(g)ͨ�����ȵ�̿�㣬����HCl��CO2������1molCl2���뷴Ӧʱ�ͷų�145kJ������д���÷�Ӧ���Ȼ�ѧ����ʽ�� ��
��2����ѧ������̫���ֽܷ�ˮ���ɵ������ڴ����������������̼��Ӧ���ɼ״�����֪H2(g)��CO(g)��CH3OH(l)��ȼ���Ȧ�H�ֱ�Ϊ��285.8kJ��mol-1����283.0kJ��mol-1�ͣ�726.5kJ��mol-1����
����̫���ֽܷ�10molˮ���ĵ�������_____________kJ��
�ڼ״�����ȫȼ������һ����̼��Һ̬ˮ���Ȼ�ѧ����ʽΪ_______ ____ ��
��3����֪��Fe2O3(s)��3C(ʯī)��2Fe(s)��3CO(g) ��H����489.0 kJ��mol-1
CO(g)��
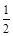 O2(g) ��CO2(g)����H����283.0 kJ��mol-1
O2(g) ��CO2(g)����H����283.0 kJ��mol-1C(ʯī)��O2(g) ��CO2(g)����H����393.5 kJ��mol-1
��4Fe(s)��3O2(g) ��2Fe2O3(s)����H�� ��
��8�֣���1��2Cl2(g)��2H2O(g)��C(s)��4HCl(g)��CO2(g) ��H����290kJ��mol-1
��2����2858 ��CH3OH(l)��O2(g)��CO(g)��2H2O(l) ��H����443.5kJ��mol-1
��3����1641.0 kJ��mol-1
��2����2858 ��CH3OH(l)��O2(g)��CO(g)��2H2O(l) ��H����443.5kJ��mol-1
��3����1641.0 kJ��mol-1
��1����Ӧ�Ļ�ѧ����ʽΪ��2Cl2(g)��2H2O(g)��C(s)��4HCl(g)��CO2(g)����2mol Cl2�μӷ�Ӧʱ�ų�������Ϊ��145kJ��2��290kJ���ʸ÷�Ӧ���Ȼ�ѧ����ʽΪ��2Cl2(g)��2H2O(g)��C(s)��4HCl(g)��CO2(g) ��H����290kJ��mol-1��
��2���ٷֽ�10molˮ���ĵ���������ֵ�ϵ���10mol H2(g)��ȫȼ��ʱ���ų���������10mol��285.8kJ��mol-1��2858kJ���ڸ���CO(g)��CH3OH(l)ȼ���ȵ��Ȼ�ѧ����ʽ�����ɸ�˹���ɿ�д���״�����ȫȼ�յ��Ȼ�ѧ����ʽ��
��3�����ø�˹���ɣ�����������Ӧ��6����һ����Ӧ��2���ڶ�����Ӧ��6���ɵ�Ŀ�귴Ӧ�����H����393.5 kJ��mol-1��6��489.0 kJ��mol-1��2��(��283.0 kJ��mol-1) ��6����1641.0 kJ��mol-1��
��2���ٷֽ�10molˮ���ĵ���������ֵ�ϵ���10mol H2(g)��ȫȼ��ʱ���ų���������10mol��285.8kJ��mol-1��2858kJ���ڸ���CO(g)��CH3OH(l)ȼ���ȵ��Ȼ�ѧ����ʽ�����ɸ�˹���ɿ�д���״�����ȫȼ�յ��Ȼ�ѧ����ʽ��
��3�����ø�˹���ɣ�����������Ӧ��6����һ����Ӧ��2���ڶ�����Ӧ��6���ɵ�Ŀ�귴Ӧ�����H����393.5 kJ��mol-1��6��489.0 kJ��mol-1��2��(��283.0 kJ��mol-1) ��6����1641.0 kJ��mol-1��

��ϰ��ϵ�д�
 Сѧͬ�����������ܾ�ϵ�д�
Сѧͬ�����������ܾ�ϵ�д�
�����Ŀ
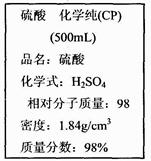
 O2��g��=== ZnO(s)����H=" -348.3" kJ��mol-1��
O2��g��=== ZnO(s)����H=" -348.3" kJ��mol-1�� 2HI(g) ��H����9.48 kJ��mol-1
2HI(g) ��H����9.48 kJ��mol-1 
