��Ŀ����
����Ŀ����Ҫ����գ�
(1)д�����з�Ӧ�ķ���ʽ��
����ϩʹ��ˮ��ɫ��_____________________________��
���Ҵ��Ĵ�������Ӧ��_________________________��
����������NaOH���Ҵ���Һ���ȣ�____________________��
�ܱ�������ķ�Ӧ��___________________________________��
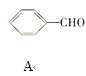
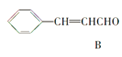
(2)0.1 molij������������������ȫȼ�գ�����CO2��H2O��0.6 mol��������ķ���ʽΪ_______������������ʹ��ˮ����������Һ��ɫ������һ�������£����Ժ�Һ�巢��ȡ����Ӧ����һ��ȡ����ֻ��һ�֣�������Ľṹ��ʽΪ___________��������_________��
���𰸡�CH2=CH2��Br2��CH2Br��CH2Br 2CH3CH2OH+O2![]() 2CH3CHO+2H2O CH3CH2Br+NaOH
2CH3CHO+2H2O CH3CH2Br+NaOH![]() CH2=CH2��+NaBr+H2O
CH2=CH2��+NaBr+H2O 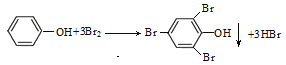 C6H12
C6H12 ![]() ������
������
��������
(1)����ϩ����ˮ�����ӳɷ�Ӧ����1,2-�������飻
���Ҵ�������������Ӧ������ȩ��
����������NaOH���Ҵ���Һ���ȣ�������ȥ��Ӧ������ϩ��
�ܱ�����Ũ��ˮ����ȡ����Ӧ���������屽�Ӱ�ɫ������
(2)����0.1mol������ȫȼ�����ɵĶ�����̼��ˮ�����ʵ�������ȷ�������ʽ�����ݸ��л���ķ�����ɼ����еĻ�ѧ�����ж���ṹ��д���ṹ��ʽ������������
(1)����ϩ����ˮ�����ӳɷ�Ӧ����1��2-�������飬��Ӧ����ʽΪCH2=CH2��Br2��CH2Br��CH2Br��
���Ҵ���O2��Cu��Ag�ȴ������ڼ����������£�����������Ӧ������ȩ��ˮ����������Ӧ�ķ���ʽΪ��2CH3CH2OH+O2![]() 2CH3CHO+2H2O��
2CH3CHO+2H2O��
����������NaOH���Ҵ���Һ���ȣ�������ȥ��Ӧ��������ϩ��NaBr��H2O����Ӧ�Ļ�ѧ����ʽΪCH3CH2Br+NaOH![]() CH2=CH2��+NaBr+H2O��
CH2=CH2��+NaBr+H2O��
�ܱ�����Ũ��ˮ����ȡ����Ӧ���������屽�Ӱ�ɫ������HBr����Ӧ�Ļ�ѧ����ʽΪ�� ��
��
(2)0.1molij������������������ȫȼ�գ�����CO2��ˮ��0.6mol����n(C)=n(CO2)=0.6mol��n(H)=2n(H2O)=2��0.6mol=1.2mol��1mol�����к���C��H�����ʵ����ֱ�Ϊ��n(C)=(0.6��0.1)mol=6mol��n(H)= (1.2��0.1)mol=12mol�����Ը����ķ���ʽΪ��C6H12��
����������ʹ��ˮ����������Һ��ɫ��������в�����̼̼˫��������һ�������£����Ժ�Һ�巢��ȡ����Ӧ����һ��ȡ����ֻ��һ�֣������ֻ��Ϊ�����飬������Ľṹ��ʽΪ��![]() ��
��

����Ŀ�������е�Ԫ�����в������Ƚ�����Ƶ���
A�����ʳ��ˮ���ռ� |
B���ϳɰ��еĴ��ϳ� |
C�����������еĴ����� |
D������еİ���ˮ̼�ữ |
����ˮɹ�ε�±ˮ�л����Ȼ�þ����±ˮΪԭ������þ��һ�й�����������ͼ��ʾ��
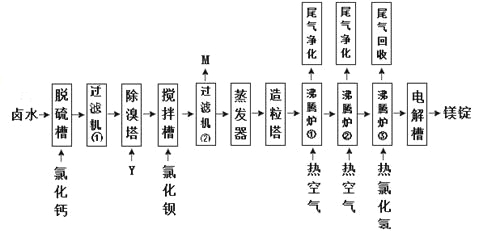
�ش��������⣺
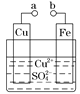
��1������ۡ�����۾������ѳ�±ˮ�еģ������ӷ��ţ���M����Ҫ�ɷ��ǣ��ѧʽ����
��2������������Ҫ�����ӷ���ʽΪ��
��3������¯����������Ҫ�����ǡ�����¯��ͨ�����Ȼ������ҪĿ���ǡ�
��4�������������ĵ缫��Ӧ����ʽΪ��
��5����������������Ϊ���ò����ֱ�����ڱ����������еġ�
����Ŀ���±���Ԫ�����ڱ���һ���֣���Ҫ����գ���Ԫ����������ѧʽ����
�� ���� | IA | IIA | IIIA | IVA | VA | VIA | VIIA | 0 |
2 | �� | �� | ||||||
3 | �� | �� | �� | �� | �� | �� | �� |
��1��д���ۺ͢�ĵ��ʷ�Ӧ����ĵ���ʽ________��
��2��![]() �����ڱ��е�λ��________������������ȫȼ�յIJ���ĵ���ʽ________��
�����ڱ��е�λ��________������������ȫȼ�յIJ���ĵ���ʽ________��
��3���ڢ���ܵ������У���ѧ���ʽϻ��õ���________���жϵ�ʵ��������________��