��Ŀ����
����Ŀ����A����Ҫ���л�����ԭ�ϣ���A�����·�Ӧ���Ʊ�һ���л�������

��֪������Ϣ��
�ٺ˴Ź������ױ���Dֻ��һ�ֻ�ѧ�������⣻
���ʻ�������ɷ������·�Ӧ��
 ����������Ҳ������Hԭ�ӣ�
����������Ҳ������Hԭ�ӣ�
��E�ڼ״�������������£�������������ˮ��Ӧ����F��
�ش��������⣺
(1)A�Ľṹ��ʽΪ _______________��A����B�ķ�Ӧ����Ϊ______________��
(2)B����C�Ļ�ѧ����ʽΪ___________________��
(3)D�Ľṹ��ʽΪ_______________________������������� _____��ԭ�ӹ�ƽ�档
(4)F�Ļ�ѧ����Ϊ_______________________��
(5)F��ͬ���칹������ͬʱ�������������Ĺ���_______��(���������칹)��
�����뱥��NaHCO3��Һ��Ӧ�������壬
����ʹBr2�����Ȼ�̼��Һ��ɫ�����к˴Ź���������ʾΪ4��壬�����Ϊ3 : 2 : 2 : 1����_______(д������һ�ֵĽṹ��ʽ)��
(6)������ ��һ������ɽ�����ϣ�����������Ϣ������Ҵ��Ʊ�������ĺϳ�·�� ___________
��һ������ɽ�����ϣ�����������Ϣ������Ҵ��Ʊ�������ĺϳ�·�� ___________
���𰸡�CH2=CHCH3 �ӳɷ�Ӧ CH3CHClCH3+NaOH![]() CH3CH(OH)CH3+NaCl CH3COCH3 6 2-����ϩ����� 8 CH2=C(CH3)CH2COOH��
CH3CH(OH)CH3+NaCl CH3COCH3 6 2-����ϩ����� 8 CH2=C(CH3)CH2COOH��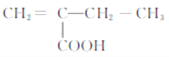
![]()
��������
F�����Ӿ۷�Ӧ�����л���������F�ṹ��ʽΪCH2=C(CH3)COOCH3��E�ͼ״������������·���������Ӧ����ˮ��Ӧ����F��E�ṹ��ʽΪ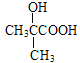 ��
��
A������������A����ʽ֪��A�ṹ��ʽΪCH2=CHCH3��A��HCl�����ӳɷ�Ӧ����B��B����ȡ����Ӧ����C��C������������Ӧ����D���˴Ź������ױ���Dֻ��һ�ֻ�ѧ������������D�ṹ��ʽΪCH3COCH3��C�ṹ��ʽΪCH3CH(OH)CH3��B�ṹ��ʽΪCH3CHClCH3��D�����ӳɷ�ӦȻ���ữ�õ�E���ݴ˽����
F�����Ӿ۷�Ӧ�����л���������F�ṹ��ʽΪCH2=C(CH3)COOCH3��E�ͼ״������������·���������Ӧ����ˮ��Ӧ����F��E�ṹ��ʽΪ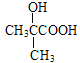 ��
��
A������������A����ʽ֪��A�ṹ��ʽΪCH2=CHCH3��A��HCl�����ӳɷ�Ӧ����B��B����ȡ����Ӧ����C��C������������Ӧ����D���˴Ź������ױ���Dֻ��һ�ֻ�ѧ������������D�ṹ��ʽΪCH3COCH3��C�ṹ��ʽΪCH3CH(OH)CH3��B�ṹ��ʽΪCH3CHClCH3��D�����ӳɷ�ӦȻ���ữ�õ�E��
(1)A�Ľṹ��ʽΪCH2=CHCH3��A����B�ķ�Ӧ����Ϊ�ӳɷ�Ӧ��
��ˣ�������ȷ������CH2=CHCH3���ӳɷ�Ӧ��
(2) B�ṹ��ʽΪCH3CHClCH3��C�ṹ��ʽΪCH3CH(OH)CH3��B����C�Ļ�ѧ����ʽΪCH3CHClCH3+NaOH![]() CH3CH(OH)CH3+NaCl��
CH3CH(OH)CH3+NaCl��
��ˣ�������ȷ������CH3CHClCH3+NaOH![]() CH3CH(OH)CH3+NaCl��
CH3CH(OH)CH3+NaCl��
(3)D�Ľṹ��ʽΪCH3COCH3�������к���C=O����������6��ԭ�ӹ�ƽ�棬
��ˣ�������ȷ������CH3COCH3 ��6 ��
(4)F�Ľṹ��ʽΪCH2=C(CH3)COOCH3����ѧ����Ϊ2-����ϩ�������
��ˣ�������ȷ������2-����ϩ�������
(5) F�Ľṹ��ʽΪCH2=C(CH3)COOCH3��F��ͬ���칹������ͬʱ��������������
�����뱥��NaHCO3��Һ��Ӧ�������壬˵�������Ȼ���
����ʹBr2�����Ȼ�̼��Һ��ɫ˵������̼̼˫����ȥ���Ȼ��������ĸ�̼ԭ���������˫�����ĸ�̼��ֱ����2�֣�C=C��C��C��C��C=C��C���ֱ����Ȼ�ȡ����Ӧ��6�ֽṹ�������������˫�����ĸ�̼�ɹǼ���![]() �����Ȼ�ȡ����Ӧ��2�ֽṹ��������������8�ֽṹ����������
�����Ȼ�ȡ����Ӧ��2�ֽṹ��������������8�ֽṹ����������
���к˴Ź���������ʾΪ4������ҷ������Ϊ3 : 2 : 2 : 1����CH2=C(CH3)C H2COOH��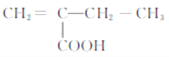
��ˣ�������ȷ������8��CH2=C(CH3)C H2COOH��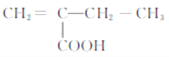 ��
��
(6) ���Ҵ�Ϊ��ʼԭ���Ʊ�������![]() ���Ҵ�������������ȩ����ȩ��HCN�����ӳɷ�Ӧ��Ȼ��ˮ���������������ᷢ�����۷�Ӧ�����ɾ�����������Ϊ
���Ҵ�������������ȩ����ȩ��HCN�����ӳɷ�Ӧ��Ȼ��ˮ���������������ᷢ�����۷�Ӧ�����ɾ�����������Ϊ ![]() ��
��
��ˣ�������ȷ������![]() ��
��

����Ŀ��̼Ԫ�������ǵ��ճ��������Ϳ�ѧ�о��ܲ��ɷ֡���ش��������⡣
��1��Al2O3���̼�Ȼ�ԭһ�Ȼ�����һ���µ���������,�÷����̶�,�豸��,����Ŀǰ�����ᳫ���ܼ��š����������Ĵ���
����ұ�������з����ķ�Ӧ�У�
(��)2Al2O3(s)+9C(s)=Al4C3(s)+6CO(g) ��H1;
(��)Al2O3(s)+Al4C3(s)+3AlCl3(g)=9AlCl(g)+3CO(g) ��H2;
(��)3AlCl(g)=AlCl3(g)+2Al(l) ��H3;
��Al2O3(s)+3C(s)= 2Al(l)+ 3CO(g) ��H4=___________________(�ú���H1����H2����H3�Ĵ���ʽ��ʾ)��
��2�����û���̿�Ļ�ԭ�Կɴ�����������β��(��������),�������·�ӦC(s)+2NO(g)![]() N2(g)+CO2(g) ��H��0��һ��������,�ܱ������е��й����ʵ�Ũ����ʱ��ı仯���±���ʾ��
N2(g)+CO2(g) ��H��0��һ��������,�ܱ������е��й����ʵ�Ũ����ʱ��ı仯���±���ʾ��
ʱ��/mim Ũ��/(mol/L) | 0 | 10 | 20 | 30 | 40 | 50 |
NO | 2.0 | 1.16 | 0.40 | 0.40 | 0.6 | 0.6 |
N2 | 0 | 0.42 | a | b | 1.2 | 1.2 |
CO2 | 0 | 0.42 | a | b | 1.2 | 1.2 |
��0~20min�ڵ�ƽ����Ӧ����v(CO2)=_______mol��L-1��min-1����һ�δﵽƽ���ƽ�ⳣ��K=__________��
��30minʱֻ�ı�ijһ��������ı������������______________ (����ĸ���)��
a�������¶� b�������¶� c����ͨ��һ������NO
d����С��������� e��������ʵĴ��� f�����������ݻ�
��3������¯�з������ӵĻ�ѧ��Ӧ,���а�����Ӧ��C(s)+CO2(g)![]() 2CO(g)��H>0����1molCO2��������̼���뵽һ����ѹ�ܱ������У���ѹǿΪP�����ﵽƽ��ʱ,��������������������¶ȵĹ�ϵ����ͼ��
2CO(g)��H>0����1molCO2��������̼���뵽һ����ѹ�ܱ������У���ѹǿΪP�����ﵽƽ��ʱ,��������������������¶ȵĹ�ϵ����ͼ��

CO2�������Ϊ86%ʱ,CO2��ת����Ϊ______________%(�������һλС��,��ͬ)��





