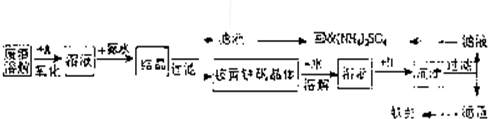
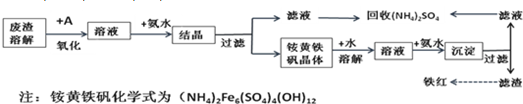
��Ŀ����
��ҵ����������������Fe2+��Fe3+�������μ�����CaO��MgO���Ʊ��ߵ��������죨Fe2O3 ���ͻ��գ�NH4��2SO4�����������������£�
��1���ڷ����ܽ����ʱ��Ӧѡ��______�ܽ⣨����ĸ����
A����ˮ������ B���������ơ����� C�����ᡡ������D������
��2������A��һ������������ҵ�����ѡ��______��ѡ��ʹ�õ��У�������Cl2��MnO2������������______
��3��������ͼ�й����ݣ�����Ϊ��ҵ����������ʱӦ���Ƶ������ǣ�______��
��4�����ᾧ����Ӧ�Ļ�ѧ����ʽΪ______��
�⣺��1���ڷ����ܽ����ʱ��Ŀ�����ܽ�������Ʊ��ߵ��������죨Fe2O3 ���ͻ��գ�NH4��2SO4�����������������ӣ�
A����ˮ�����ܽ�������ܳ��������Ӻ��������ӣ���A��ѡ��
B������������Һ���������Ӻ��������Ӳ����ܽ������ơ�����þ����B��ѡ��
C�������ܽ������ƺ�����þ�������������Ӳ��ܳ�ȥ����C��ѡ��
D���������ܽ������ƺ�����þ���Ҳ������������ӣ���D���ϣ�
�ʴ�Ϊ��D��
��2����������Ҫ�������������ӱ�����ȡ�������ж�����Ⱦ����������������������ˮ�Ĺ��壬������Դ�ḻ���ɱ��ͣ����������ʣ���������Ⱦ��
�ʴ�Ϊ�������� ԭ����Դ���ף��ɱ��ͣ���������Ⱦ�����������ʣ�
��3������ͼ���֪��80��Cʱ ��ҺPH=1.5����ʱ�������ӵ���������90%���ϣ�ʱ����4Сʱ���ң�
�ʴ�Ϊ����Һ�¶�Ϊ80�棬pHΪ1.5��ʱ��Ϊ4Сʱ���ң�
��4�������ᾧ����Ӧ���������ڰ�ˮ�з�Ӧ����炙�����������泥�����ԭ���غ���ƽд����Ӧ�Ļ�ѧ����ʽΪ3Fe2��SO4��3+12NH3?H2O=��NH4��2Fe6��SO4��4��OH��12��+5 ��NH4��2SO4��
�ʴ�Ϊ��3Fe2��SO4��3+12NH3?H2O=��NH4��2Fe6��SO4��4��OH��12��+5 ��NH4��2SO4��
��������1�����ݷ����ɷֽ���ᴿ������ɷ����жϣ�
��2����������Ҫ������ �����������ӣ�������Դ�ḻ����Ч��߲�������Ⱦ�Ȼش�
��3������ͼ��������������ӵ��������������Һ�¶�Ϊ80�棬pHΪ1.5��
��4����������ͼ���ת����ϵ�е����ʡ���Ӧ�Լ��͵õ����ﻯѧʽ������ԭ���غ���ƽд����
���������⿼���������Ʊ����ᴿ��ʵ�����̷����жϣ�ͼ�����Ӧ�ã���ѧ����ʽ��д��������Ŀ��Ϣ������Ҫ��ϸ���⣬��Ŀ�Ѷ��еȣ�
A����ˮ�����ܽ�������ܳ��������Ӻ��������ӣ���A��ѡ��
B������������Һ���������Ӻ��������Ӳ����ܽ������ơ�����þ����B��ѡ��
C�������ܽ������ƺ�����þ�������������Ӳ��ܳ�ȥ����C��ѡ��
D���������ܽ������ƺ�����þ���Ҳ������������ӣ���D���ϣ�
�ʴ�Ϊ��D��
��2����������Ҫ�������������ӱ�����ȡ�������ж�����Ⱦ����������������������ˮ�Ĺ��壬������Դ�ḻ���ɱ��ͣ����������ʣ���������Ⱦ��
�ʴ�Ϊ�������� ԭ����Դ���ף��ɱ��ͣ���������Ⱦ�����������ʣ�
��3������ͼ���֪��80��Cʱ ��ҺPH=1.5����ʱ�������ӵ���������90%���ϣ�ʱ����4Сʱ���ң�
�ʴ�Ϊ����Һ�¶�Ϊ80�棬pHΪ1.5��ʱ��Ϊ4Сʱ���ң�
��4�������ᾧ����Ӧ���������ڰ�ˮ�з�Ӧ����炙�����������泥�����ԭ���غ���ƽд����Ӧ�Ļ�ѧ����ʽΪ3Fe2��SO4��3+12NH3?H2O=��NH4��2Fe6��SO4��4��OH��12��+5 ��NH4��2SO4��
�ʴ�Ϊ��3Fe2��SO4��3+12NH3?H2O=��NH4��2Fe6��SO4��4��OH��12��+5 ��NH4��2SO4��
��������1�����ݷ����ɷֽ���ᴿ������ɷ����жϣ�
��2����������Ҫ������ �����������ӣ�������Դ�ḻ����Ч��߲�������Ⱦ�Ȼش�
��3������ͼ��������������ӵ��������������Һ�¶�Ϊ80�棬pHΪ1.5��
��4����������ͼ���ת����ϵ�е����ʡ���Ӧ�Լ��͵õ����ﻯѧʽ������ԭ���غ���ƽд����
���������⿼���������Ʊ����ᴿ��ʵ�����̷����жϣ�ͼ�����Ӧ�ã���ѧ����ʽ��д��������Ŀ��Ϣ������Ҫ��ϸ���⣬��Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ