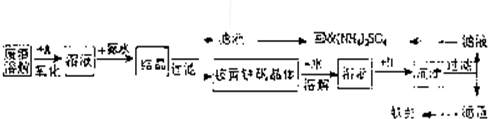
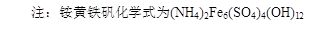��Ŀ����
��ҵ����������������Fe2+��Fe3+�������μ�����CaO��MgO���Ʊ��ߵ���������(Fe2O3 )�ͻ���(NH4)2SO4�����������������£�
��1���ڷ����ܽ����ʱ��Ӧѡ��__________�ܽ⣨����ĸ����
A����ˮ B���������� C������ D������
��2������A��һ������������ҵ�����ѡ�� ����ѡ��ʹ�õ��У�������Cl2��MnO2������������ ��
��3��������ͼ�й����ݣ�����Ϊ��ҵ����������ʱӦ���Ƶ������ǣ� ��
��4�����ᾧ����Ӧ�Ļ�ѧ����ʽΪ_________________ ____________________��
��5���������顰��Һ���к���NH4����ʵ�鷽���� ��
��1��D�� 2�� ����2��������2�֣��� ԭ����Դ���ף��ɱ��ͣ���������Ⱦ�����������ʡ���2�֣����������㼴�ɣ�
��3����Һ�¶ȿ�����80�棨1�֣���pH������1.5��1�֣�������ʱ��Ϊ4Сʱ���ң�2�֣�
��4��3Fe2(SO4)3 �� 2NH3��H2O �� 10H2O�� (NH4)2Fe6(SO4)4(OH)12��+5H2SO4 ��3�֣�
��5��ȡ������Һ���Թ��У����Թ��м���������Ũ����������Һ�����ȡ���ʪ��ĺ�ɫʯ����ֽ�����Թܿڴ�����ɫʯ����ֽ����ɫ��˵����Һ�к���NH4+����3�֣�
����:��