��Ŀ����
����Ŀ��������(C6H5COOHʽ����122���۵�122��4�棬�ܶ�Ϊ1.2659g��cm-3)��һ��һԪ�л����ᣬ����ˮ�������Ҵ���ʵ�������ɼױ�(ʽ����92���ܶ�Ϊ0.8669 g��cm-3)�Ʊ��������ʵ�����£�

��һ������18.4g�ױ��������ữ��KMnO4��Һ������ͼ������ƿ�У����ȱ��ַ�Ӧ����Һ�¶���90����������Ӧ������
�ڶ���������Ӧ����Һ���ˣ���Һ��Ũ�����ữ�����˵ôֲ�Ʒ��
���������ֲ�Ʒ��ˮϴ��2��3�Σ���������ù���23.4g��
��ش��������⣺
(1)����������Ϊ_________________��
(2)��һ��������Ӧ�Ļ�ѧ����ʽΪ____________________________��
(3)�ڶ����г��˵��ŵ���____________________________________��
(4)������֤��ϴ�Ӹɾ��ķ�����_________________________________��
�������ѷ�����________ (�����)
a.��������Ȼ���b����ˮԡ�ϸ���c��ֱ�Ӽ��ȸ���
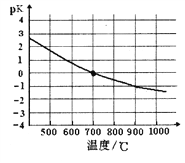
(5)����������֪������IJ���Ϊ_____________��
(6)ijѧ��Ϊ�ⶨ������ĵ���ƽ�ⳣ�����ʵ�����£������£���a mol�������b mol KOH��ϼ�ˮ�γ�2L��Һ�������Һ��pH=7�����ú�a��b�Ĵ���ʽ��ʾ���������Ka=____________������Һ�е�����Ũ�ȴ�С��ϵΪ____________________��
���𰸡� �¶ȼ� 5C6H5CH3+6KMnO4+9H2SO4��5C6H5COOH+3K2SO4+6MnSO4+14H2O �����ٶȿ죬�õ��Ĺ���ˮ���� ȡ���һ�ε�ϴ��Һ�еμ���������Һ������ͽ���ɣ���˵������ϴ�Ӹɾ��� b 96����9-9% b��10-7/(a-b) c(K+)=c(C6H5COO-)>c(H+)=c(OH-)
��������(1)�������������֪����������Ϊ�¶ȼơ�(2)��һ�������ķ�Ӧ�Ǽױ��������ﱽ���ᣬ��Ӧ�Ļ�ѧ����ʽΪ5C6H5CH3+6KMnO4+9H2SO4��5C6H5COOH+3K2SO4+6MnSO4+14H2O��(3)�ڶ����г��˵��ŵ��ǹ����ٶȿ죬�õ��Ĺ���ˮ���١�(4)���ڵڶ�������Ũ�������ԣ���˿���ͨ���������������жϵ�����֤��ϴ���Ƿ�ɾ���������ȡ���һ�ε�ϴ��Һ�еμ���������Һ������ͽ���ɣ���˵������ϴ�Ӹɾ��ˣ���������۵㳬��100�棬Ϊ�˼ӿ������̣�������ģ��������ѷ����Ƿ�ˮԡ�ϸ����ѡb��(5) 18.4g�ױ������ʵ�����0.2mol�����������ɱ�������0.2mol��������0.2mol��122g/mol��24.4g����˱�����IJ���Ϊ23.4g/24.4g��100%��96%��(6)���������غ��֪��Һ��c(C6H5COO-)��c(C6H5COOH)��0.5a�����������bmol����c(C6H5COO-)��0.5bmol�����Ա������Ka=![]() ��b��10-7/(a-b)��
��b��10-7/(a-b)��
���ݵ���غ��֪����Һ�е�����Ũ�ȴ�С��ϵΪc(K+)=c(C6H5COO-)>c(H+)=c(OH-)��