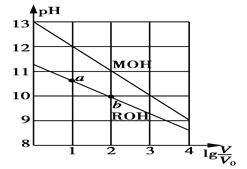
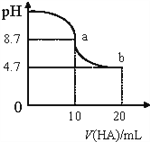
��Ŀ����
����Ŀ��һ�������г��õĴ�����ҺŨ��Ϊ 0.5 mol/L��ijͬѧ���ô����ĩ���Ƹ���Һ 480 mL ���á���ش�����������⣺
��1�����ձ����������⣬һ������Ҫ�õ��IJ���������____________
��2�����㣺��Ҫ��ȡ������������Ϊ__________ g
��3������ʱ����ȷ�IJ���˳���ǣ�ÿ�����ֻ��һ�Σ�_______________
A��������ˮϴ���ձ� 2 �Ρ�3 �Σ�ϴ��Һ��ע������ƿ����
B����ʢ�д��������ձ��м�������ˮ�ܽ�
C�����ָ������µ���Һ�ز�����ע������ƿ��
D��������ƿ���������µߵ�ҡ��
E�����ý�ͷ�ιܼ�ˮ��ʹ��Һ��Һ��ǡ����̶�����ƽ
F������������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶� 1 cm��2 cm ��
��4�����лᵼ��������Һʵ��Ũ��ƫ�ߵ���____________
a���� C ������δ�ָ������¼�ע������ƿ
b���� D ������ɺ���Һ����ڿ̶���
c���� E �����и��Ӱ�Һ��
��5��������������Һ�������� 100 mL 0.2 mol/L �Ĵ�����Һ������ȡ��������Һ�����Ϊ_________mL
���𰸡���ͷ�ιܡ�500mL����ƿ 26.5g BCAFED ac 40.0����40��
��������
��1������һ�����ʵ���Ũ�ȵ���Һ���Ʋ���ѡ��������
��2������m=cVM������Ҫ���ʵ�������
��3������һ�����ʵ���Ũ�ȵ���Һ�IJ���Ϊ�����㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��
��4��������������Һ�����ʵ����ʵ���Ũ�ȵ�Ӱ�죬����c=![]() ���з�����
�������
��5������ϡ���������ʵ����ʵ���������м��㡣
��1������һ�����ʵ���Ũ�ȵ���Һ��Ҫ�õ�����ƽ��ҩ�ס��ձ���������������ƿ����ͷ�ιܡ���������480mL��Һ����Ҫ500mL����ƿ�����л�ȱ�ٵIJ�������Ϊ����ͷ�ιܣ�500mL����ƿ��
��2����������480mL��Һ��Ҫ��500mL����ƿ�������500mL���м��㡣m=cVM=0.5 mol/L��0.5L��106g/mol=26.5g��
��3������һ�����ʵ���Ũ�ȵ���Һ�IJ���Ϊ�����㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ����Ϊ��BCAFED��
��4��a���� C ������δ�ָ������¼�ע������ƿ����ȴ����Һ������٣�Ũ��ƫ�ߣ�a���ϡ�
b���� D ������ɺ���Һ����ڿ̶��ߣ�������ȷ������ҺŨ����Ӱ�죬b�����ϡ�
c���� E �����и��Ӱ�Һ�棬������Һ�����С����ҺŨ��ƫ�ߣ�c���ϡ�
��5����Ҫ��Һ���ΪV��������Һϡ���������ʵ����ʵ�������ã�100 mL��0.2 mol/L=V��0.5 mol/L��V=40mL��