��Ŀ����
pC����pH����ָ��ϡ��Һ���������ʵ���Ũ�ȵij��ö�����ֵ����ij��Һ���ʵ�Ũ��Ϊ��1��10-3mol��L-1�������Һ�����ʵ�pC=3�����б�����ȷ����
A��ij�¶����κε���ʵ�ˮ��Һ�У�pC(H+) +pC(OH-)=14
B��0.01mol/L��CaCl2��Һ���μӴ�����Һ���μӹ�����pC(Ca2+)��С
C����0.01mol/L������ζ�ijŨ�ȵ�NaOH��Һ���ζ�������pC(H+)������
D��ij�¶��£�AB���������ӻ������Ksp=1.0��10-10, �䱥����Һ��pC(A+) +pC((B-)=10
���𰸡�
D
��������
���������pC(H+) +pC(OH-)=14ֻ���ڳ����²ų�������A����0.01mol/L��CaCl2��Һ���μӴ�����Һ��c(Ca2��)��С������pC(Ca2+)������B������0.01mol/L������ζ�ijŨ�ȵ�NaOH��Һ���ζ�������c(H+)��������pC(H+)��С��Cѡ�����ij�¶��£�AB���������ӻ������Ksp=1.0��10-10, c(A+)��c(B-)��1.0��10-5��pC(A+) ��pC((B-)=5������pC(A+) +pC((B-)=10��Dѡ����ȷ��
���㣺����ѧ��������Ϣ��������Ϣ��������

��ϰ��ϵ�д�
 �������¿��ÿ�ʱ��ҵϵ�д�
�������¿��ÿ�ʱ��ҵϵ�д� Ӣ�żƻ�ͬ����ʱ��Чѵ��ϵ�д�
Ӣ�żƻ�ͬ����ʱ��Чѵ��ϵ�д�
�����Ŀ
pC����pH����ָ��ϡ��Һ���������ʵ���Ũ�ȵij��ö�����ֵ����ij��Һ���ʵ�Ũ��Ϊ��1��10-3mol?L-1�������Һ�����ʵ�pC=3�����б�����ȷ���ǣ�������
| A��ij�¶����κε���ʵ�ˮ��Һ�У�pC��H+��+pC��OH-��=14 | B��0.01mol/L��CaCl2��Һ���μӴ�����Һ���μӹ�����pC��Ca2+����С | C����0.01mol/L������ζ�ijŨ�ȵ�NaOH��Һ���ζ�������pC��H+�������� | D��ij�¶��£�AB���������ӻ������Ksp=1.0��10-10���䱥����Һ��pC��A+��+pC��B-��=10 |
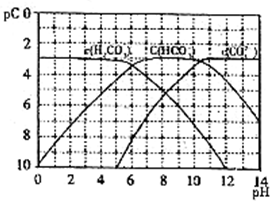 pC����pH����ָ��ϡ��Һ���������ʵ���Ũ�ȵij��ö�����ֵ����ij��Һ���ʵ�Ũ��Ϊ1��10-3mol/L������Һ�и����ʵ�pC=-lg10-3=3����֪H2CO3��Һ�д�������ƽ�⣺
pC����pH����ָ��ϡ��Һ���������ʵ���Ũ�ȵij��ö�����ֵ����ij��Һ���ʵ�Ũ��Ϊ1��10-3mol/L������Һ�и����ʵ�pC=-lg10-3=3����֪H2CO3��Һ�д�������ƽ�⣺ �������£��÷�Ӧ�ﵽ��ѧƽ��ʱ��������Ũ��Ϊ
�������£��÷�Ӧ�ﵽ��ѧƽ��ʱ��������Ũ��Ϊ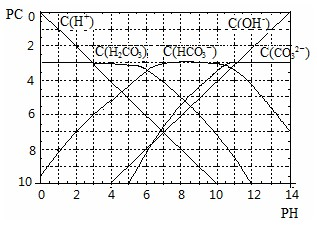
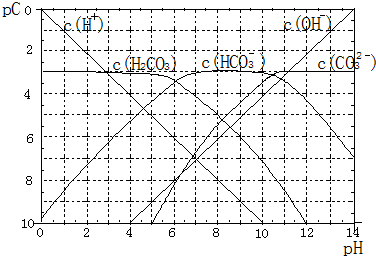
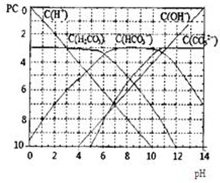 pC����pH����ָ��ϡ��Һ�У��������ʵ���Ũ�ȵij��ö�����ֵ����ij��Һ���ʵ�Ũ��Ϊ��1��10-3mol/L�������Һ�����ʵ�pC=-lg1��10-3=3��ͼΪH2CO3�ڼ���ǿ���ǿ����Һ��ƽ��ʱ��Һ�����ֳɷֵ�pC-pHͼ����ش��������⣺
pC����pH����ָ��ϡ��Һ�У��������ʵ���Ũ�ȵij��ö�����ֵ����ij��Һ���ʵ�Ũ��Ϊ��1��10-3mol/L�������Һ�����ʵ�pC=-lg1��10-3=3��ͼΪH2CO3�ڼ���ǿ���ǿ����Һ��ƽ��ʱ��Һ�����ֳɷֵ�pC-pHͼ����ش��������⣺