��Ŀ����
����Ŀ����������Ҫ��ȼú��Ⱦ������ŷţ��о�Эͬ�������������Ǵ���������
�����ӻ�ѧ�����ܽ�ʾ����ѧ��Ӧ������
SO2��ClO2����̬��Ӧ�У���Ӵ�λ�ò�ͬ���γ��˲�ͬ��Ӧ·������Ӧ�и�פ�㣨TSΪ����̬��RC��IM��PCΪ�м�����Գ�ʼ��Ӧ���������ϵ��ͼ1��ʾ
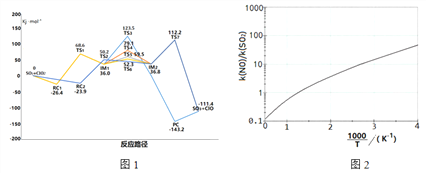
��1��д���ù��̵��Ȼ�ѧ����ʽ��____________________��
��2��ͼ��IM1��IM2����������̬_____��·����Ӧ������������ԭ����______��
��3����ͼ������ָ���÷�Ӧ��������·����____________
��4������ClOҲ��ǿ�����ԣ����Լ�������SO2����д���÷�Ӧ�Ļ�ѧ����ʽ______��
��5��ClO2�ֱ�����NO��SO2��Ӧ���ʳ���֮�����¶ȹ�ϵ��ͼ2��ʾ������֪���¶����ߣ�ClO2����NO��Ӧ����_______��
��ģ�����������Ϊ��0.03%NO��0.1%SO2��6.0%O2��8.0%H2O����70���£���n��C1O2��:n��NO��������������Ϊ������ʱ��SO2��NO��ת���ʱ仯��ͼ3��ʾ��
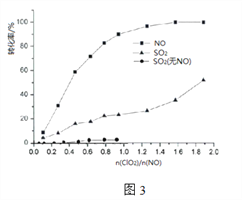
��6����NO����ʱ��ClO2����SO2______���������������������������������Ŀ���ԭ����________________��
���𰸡� SO2(g)+ClO2(g)=SO3(g)+ClO(g) H=-111.4KJ/mol TS3 ��ܸ�ù���̬����Ҫ�������ߣ����������𰸣� 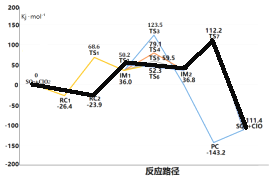 ClO+SO2=SO3+Cl��д��Cl2Ҳ���֣� ���ͣ���С�� �� NO������SO2�д����ã���NO2������SO2��
ClO+SO2=SO3+Cl��д��Cl2Ҳ���֣� ���ͣ���С�� �� NO������SO2�д����ã���NO2������SO2��
�����������������(1).����ͼʾ��1molSO2(g)��1molClO2(g)��ȫ��Ӧ����1molSO3(g)��1molClO(g)�ų�111.4 KJ��������(2)��ͼ���֪IM1��IM2������TS3�Ļ�����Ӧ��������������3�����ԽС��Ӧ����Ҫ������Խ������4��ClO��������SO2����SO3��Cl����5����ͼ2��֪���ź�����1000/T���������߳ʵ������ƣ����Ե�T����ʱ��1000/T�ڼ�С��������Ӧ���ݼ���(6)����ͼ3���Կ�������NOʱSO2�����������ʿ죬��Ҫԭ�������NO���������
������(1).����ͼʾ��1molSO2(g)��1molClO2(g)��ȫ��Ӧ����1molSO3(g)��1molClO(g)�ų�111.4 KJ�����������Ը÷�Ӧ���Ȼ�ѧ����ʽ��(1). SO2(g)+ClO2(g)=SO3(g)+ClO(g) H= -111.4KJ/mol��(2)��ͼ���֪IM1��IM2������TS3�Ļ�����Ӧ���������������Ծ�������̬TS3��·����Ӧ������������3�����ԽС��Ӧ����Ҫ������Խ�ͣ��������·���� ����4��ClO��������SO2����SO3��Cl����Ӧ����ʽ��ClO+SO2=SO3+Cl����5����ͼ2��֪���ź�����1000/T���������߳ʵ������ƣ����Ե�T����ʱ��1000/T�ڼ�С��������Ӧ���ݼ���������֪���¶����ߣ�ClO2����NO��Ӧ����������(6)����ͼ3���Կ�������NOʱSO2�����������ʿ죬ClO2����SO2Խ������Ҫԭ�������NO������á�
����4��ClO��������SO2����SO3��Cl����Ӧ����ʽ��ClO+SO2=SO3+Cl����5����ͼ2��֪���ź�����1000/T���������߳ʵ������ƣ����Ե�T����ʱ��1000/T�ڼ�С��������Ӧ���ݼ���������֪���¶����ߣ�ClO2����NO��Ӧ����������(6)����ͼ3���Կ�������NOʱSO2�����������ʿ죬ClO2����SO2Խ������Ҫԭ�������NO������á�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�

