��Ŀ����
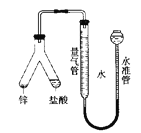
����Ŀ����ʳ�ִ���������(A1P)Ѭ��ɱ�棬A1P��ˮ������ǿ��ԭ�Ե�PH3���塣���ұ��涨��ʳ������(��PH3��)�IJ�����������0.05 mgkg-1ʱΪ�ϸ�ijС��ͬѧ����ͼ��ʾʵ��װ�ú�ԭ���ⶨij��ʳ��Ʒ�е�����IJ�������C�м���100 gԭ����E �м���20.00mL2.50��lO-4molL-1KMnO4��Һ��H2SO4�ữ)��C�м�������ˮ����ַ�Ӧ�����������Ʊ���Һ�ζ�E�е���Һ��
(1)װ��A�е�KMn04��Һ��������_____��
(2)װ��B��ʢװ����ûʳ����ļ�����Һ���տ����е�O2����ȥ����װ�ã����õ�����IJ�����___(����ƫ�{����ƫ��������������)��
(3)װ��E��PH3���������ᣬMnO4-����ԭΪMn2+��д���÷�Ӧ�����ӷ���ʽ��__________��
(4)�ռ�װ��E�е�����Һ����ˮϡ����250 mL����ȡ���е�25.00 mL����ƿ�У� ��4.0��lO-5molL-1��Na2SO3����Һ�ζ�������Na2SO3����Һ20.00mL����Ӧԭ���� S02-+Mn04-+H+��S042-+Mn2++H20(δ��ƽ)ͨ�������жϸ���Ʒ�Ƿ�ϸ�(д���������)_______��
���𰸡����տ����еĻ�ԭ�����壬��ֹ�����pH3�IJⶨ ƫ�� 5PH3+8Mn04-+24H+=5H3PO4+8Mn2++12H2O 0.382 5mg>0.05mg�����Բ��ϸ�
��������
(1) KMnO4��Һ��ǿ�����ԣ�PH3��ǿ��ԭ�ԣ�
(2)����������һ����PH3���ζ����ĵ��������Ʊ���Һƫ�٣����õ�����IJ�����ƫ�ͣ�
(3) �ɵ�ʧ�����غ㡢ԭ���غ㡢����غ��д����ȷ�Ļ�ѧ����ʽ��
(4)�ȼ���Na2SO3����Һ���ĵĸ�����ص����ʵ��������ɸ�������ܵ����ʵ�����ȥNa2SO3����Һ���ĵĸ�����ص����ʵ����������PH3���ĵĸ�����ص����ʵ��������������ʳ������(��PH3��)�IJ�������
(1) KMnO4��Һ��ǿ�����ԣ�PH3��ǿ��ԭ�ԣ�װ��A�е�KMnO4��Һ�����������տ����еĻ�ԭ�����壬��ֹ�����PH3�IJⶨ��
(2)װ��B��ʢװ����ûʳ����ļ�����Һ���տ����е�O2����ȥ����װ�ã�����������һ����PH3������ʣ�µ�KMnO4�࣬�ζ����ĵ��������Ʊ���Һƫ�٣����õ�����IJ�����ƫ�ͣ�
(3)װ��E��PH3���������ᣬMnO4-����ԭΪMn2+���ɵ�ʧ�����غ㡢ԭ���غ㡢����غ��֪���÷�Ӧ�����ӷ���ʽΪ��5PH3+8MnO4-+24H+=5H3PO4+8Mn2++12H2O��
(4) �ζ��ķ�Ӧԭ���� 5SO32-+2MnO4-+16H+=5SO42-+2Mn2++8H2O��Na2SO3����Һ���ĵĸ�����ص����ʵ���=![]() =3.2
=3.2![]() mol��������PH3���ĵĸ�����ص����ʵ���=
mol��������PH3���ĵĸ�����ص����ʵ���=![]() 2.50��lO-4molL-1
2.50��lO-4molL-1![]() 3.2
3.2![]() mol=1.8
mol=1.8![]() mol��PH3�����ʵ���=1.8
mol��PH3�����ʵ���=1.8![]() mol
mol![]() =1.125
=1.125![]() mol����ʳ������(��PH3��)�IJ�����=
mol����ʳ������(��PH3��)�IJ�����=![]() =0.382 5mgkg-1��0.382 5mgkg-1>0.05 mgkg-1�����Բ��ϸ�
=0.382 5mgkg-1��0.382 5mgkg-1>0.05 mgkg-1�����Բ��ϸ�

 �Ͻ�ƽСѧ��������ϵ�д�
�Ͻ�ƽСѧ��������ϵ�д� �Ƹ������������ϵ�д�
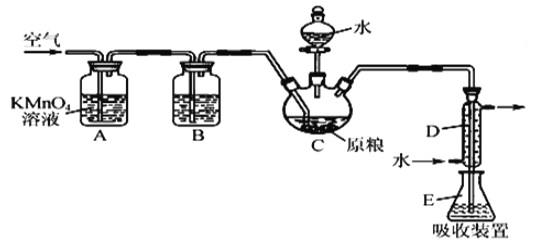
�Ƹ������������ϵ�д�����Ŀ�������ⶨ1mol�����������ʵ���У�����ͨ��ѡ��IJ�����������������Ӧ��þ��ϡ���ᷴӦ��ͼ�е�A��B��C���������������Ħ������ⶨװ�ã�

(1)Cװ�õ�������___________________��
(2)A��B��Cװ�ýӿڵ�����˳����_________________��
(3)��װ����ȷ���Ӻ������������Լ�飿_________________��
(4)��ʵ������������Ͳ��������Ҫ��¼���ǵ�____�γ������������
(5)�±���ijͬѧ��¼��ʵ�����ݣ��¶ȣ�25�棬��ѹ��101.3kPa
ʵ����� | þ������(g) | �������(mL) | Cƿ����(mL) | �����������(mL) |
1 | 0.115 | 10.0 | 124.8 | 7.0 |
2 | 0.110 | 10.0 | 120.7 | 6.2 |
��������ʵ��1mol�����������ƽ��ֵ=____L(����һλС����þ�����ԭ������Ϊ24.3)��
(6)��֪ʵ���¶��£�1mol���������������ֵΪ24.5L��ʵ�����=____%(������λ��Ч����)��
����Ŀ�����б�Ŵ���Ԫ�����ڱ��е�һ����Ԫ�أ��û�ѧʽ��Ԫ�ط��Żش��������⣺
| ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
һ | ||||||||
�� | �� | �� | ||||||
�� | �� | �� | �� | �� | �� | |||
�� | �� | �� | �� |
(1)�١��ۡ��ݵ�����������ˮ���������ǿ����_______(�ѧʽ����ͬ)��
(2)�ڡ��ۡ����γɵļ������Ӱ뾶�ɴ�С��˳����______________��
(3)�ٺ͢������������Ӧ��ˮ���ﻯѧʽΪ________��________���ٺ͢���Ԫ���γɵĻ�����Ļ�ѧʽΪ________���û��������Һ��Ԫ�آߵĵ��ʷ�Ӧ����������ʽΪ_______________________��
(4)�ߡ��ࡢ������Ԫ���γɵ���̬�⻯�����ȶ�����________(�ѧʽ����ͬ)������Ԫ�طǽ�������ǿ������˳��Ϊ________��