��Ŀ����
13����֪��ȩ��������ȫȼ�յ��Ȼ�ѧ����ʽ������ʾ����HCHO��g��+O2��g����CO2��g��+H2O��l��+571kJ
��CH2COOH��g��+2O2��g����2CO2��g��+2H2O��l��+926kJ
����˵����ȷ���ǣ�������
| A�� | 1mol HCHO��g����ȫȼ��������̬ˮʱ���ȴ���571kJ | |
| B�� | 1mol CH3COOH��l����ȫȼ������Һ̬ˮʱ���ȴ���926kJ | |
| C�� | ��ͬ�����£���������HCHO��g����CH3COOH��g������������ȣ�ǰ�߸��� | |
| D�� | ��ͬ�����£���������HCHO��g����CH3COOH��g����ȫȼ�գ����߷��ȸ��� |
���� A�����ݢٷ�Ӧ������Һ̬ˮ�仯Ϊ��̬ˮ��Ҫ����������
B�����ݷ�Ӧ����������̬�仯ΪҺ̬���ȷ�����
C����ȩ�������Dz�ͬ���ʣ������ķ�Ӧ������ϵ��ͬ�������������ܱȽϣ�
D���������ļ�ȩ�����ᣬ�������ʵ�������Ȼ�ѧ����ʽ���㷴Ӧ�ų��������Ƚϣ�
��� �⣺A��HCHO��g��+O2��g����CO2��g��+H2O��l��+571kJ��Һ̬ˮ�仯Ϊ��̬ˮ��Ҫ����������1mol HCHO��g����ȫȼ��������̬ˮʱ����С��571kJ����A����
B��CH3COOH��g��+2O2��g����2CO2��g��+2H2O��l��+926kJ��������̬�仯ΪҺ̬���ȣ�1mol CH3COOH��l����ȫȼ������Һ̬ˮʱ���ȴ���926kJ����B��ȷ��
C����ȩ�������Dz�ͬ���ʣ������ķ�Ӧ������ϵ��ͬ�������������ܱȽϣ���C����
D����ͬ�����£���������HCHO��g����CH3COOH��g����ȫȼ�գ�
��HCHO��g��+O2��g����CO2��g��+H2O��l��+571kJ
1 571KJ
$\frac{1}{30}$ 19.03KJ
��CH2COOH��g��+2O2��g����2CO2��g��+2H2O��l��+926kJ
1 926KJ
$\frac{1}{60}$ 15.43KJ
ǰ�߷��ȸ��࣬��D����
��ѡB��
���� ���⿼�����Ȼ�ѧ����ʽ��д�����ʱ仯�����еķ�Ӧ�����仯�����жϣ����ջ����ǽ���ؼ�����Ŀ�ϼ�

| A�� | �ֻ����� | B�� | ��ѧ���� | C�� | ʯӢ���� | D�� | ��ɫ���� |
| A�� | �������ķ�����֮��Ϊ2��1��2 | |
| B�� | ��������ѹǿ���ٷ����仯 | |
| C�� | ��������ƽ����Է����������ٷ����仯 | |
| D�� | ���������ܶȲ��ٷ����仯 |
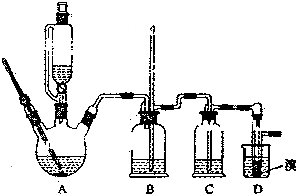 ʵ�����Ʊ�1��2-��������ķ�Ӧԭ�����£�
ʵ�����Ʊ�1��2-��������ķ�Ӧԭ�����£�CH3CH2OH$��_{170��}^{H_{2}So_{4}��Ũ��}$CH2=CH2+H2O��CH2=CH2+Br2��BRCH2B
���ܴ��ڵ���Ҫ����Ӧ�У��Ҵ���Ũ����Ĵ�������140����ˮ�������ѣ�Ũ������Ҵ�����ΪCO2�ȣ�
������������������Ҵ��Ʊ�1��2-���������װ����ͼ��ʾ��
�й������б����£�
| �Ҵ� | 1��2-�������� | ���� | |
| ״̬ | ��ɫҺ�� | ��ɫҺ�� | ��ɫҺ�� |
| �ܶ�/g•cm3 | 0.79 | 2.2 | 0.71 |
| �е�/��C | 78.5 | 132 | 34.6 |
| �۵�/��C | -130 | 9 | -116 |
��1��Aװ���Ϸ�ʹ�õ�Һ©�����ŵ��ǣ�����©����Һ��˳�����£��������һ��ʱ��������ǼӴ�Ƭ��Ӧ�ò�ȡ����ȷ������B������ȷ�𰸱�ţ���
A���������� B����ȴ�� C�����貹�� D����������
��2��Bװ�õ�������ƽ��ѹǿ�����װ���Ƿ���������
��3����װ��C��Ӧ����C������ȷѡ��ǰ����ĸ������Ŀ�������շ�Ӧ�����SO2��CO2�������壮
a��ˮ b��Ũ���� c������������Һ d������̼��������Һ
��4���жϸ��Ʊ���Ӧ�Ѿ�������������������ɫ��ȫ��ȥ��
��5��Dװ�þ�֧�Թ���������ˮ����Һ�壨�ٶ�������ͬ�����������ŵ����ɵ�1��2-���������ˮ�ֲ㣬ˮ���ϲ���Һ�����ã���ֹ��Ʒ�ӷ���
��6����Ӧ������Ӧ����ˮ��ȴװ��D������ҪĿ������ϩ���巴Ӧʱ���ȣ���ȴ�ɱ�����Ĵ����ӷ������ֲ��ܹ�����ȴ�����ñ�ˮ������ԭ����1��2-������������̵�ϵͣ�9�棩��������ȴ��ʹ�����̶�ʹ��·������
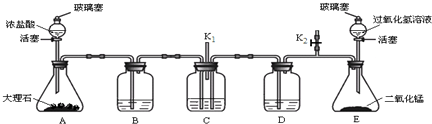
| A�� | ����ʯ���ڴ�����Һ��CaCO3+2H+�TCa2++CO2��+H2O | |
| B�� | ��FeBr2��Һ��ͨ������������2Fe2++4Br++3Cl2�T2Fe3++2Br2+6Cl- | |
| C�� | NH4HCO3���ڹ�����NaOH��Һ�У�HCO3-+OH-�TCO32-+H2O | |
| D�� | ��������KAl��SO4��2•12H2O��Һ�еμ�Ba��OH��2��Һ��ǡ��ʹSO42-������ȫ��2Al3++3SO42-+3Ba2++6OH-�T2Al��OH��3��+3BaSO4�� |