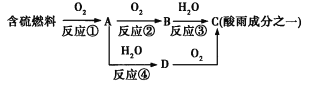
ЬтФПФкШн
ЁОЬтФПЁПгаЛњЮяWдквНвЉКЭаТВФСЯЕШСьгђгаЙуЗКгІгУЁЃWЕФвЛжжКЯГЩТЗЯпШчЯТ:

вбжЊВПЗжаХЯЂШчЯТ:
ЂЁ) 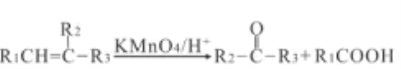
ЂЂ) 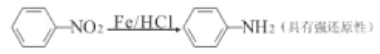
ЂЃ) 
ЂЄ) RЪЧБНЕФЭЌЯЕЮяЃЌФІЖћжЪСПЮЊ106g/mol,RЕФвЛТШДњЮяга5жжВЛЭЌНсЙЙ: TЮЊвЛЯѕЛљЛЏКЯЮяЃЛHЗжзгдкКЫДХББеёЧтЦзЩЯга5ИіЗхЁЃ
ЂЅЃЉ1molYЭъШЋЗДгІЩњГЩ2molZ,ЧвдкМгШШЬѕМўЯТZВЛФмКЭаТжЦЧтбѕЛЏЭзЧвКЗДгІЃЌ
ЂІЃЉБНМзЫсЕФЫсадБШбЮЫсШѕЃЌБНМзЫсгыХЈСђЫсЃЌХЈЯѕЫсдкМгШШЬѕМўЯТжївЊЗЂЩњМфЮЛШЁДњЗДгІ
ЧыЛиД№ЯТСаЮЪЬт:
ЃЈ1ЃЉXЕФЛЏбЇУћГЦЪЧ_______ЃЛZжаЙйФмЭХУћГЦЮЊ________ЃЛ
ЃЈ2ЃЉTЕФНсЙЙМђЪНЮЊ_____;ЭМЪОжаXзЊЛЏЮЊYЕФЗДгІРраЭЪЧ______
ЃЈ3ЃЉZКЭHдквЛЖЈЬѕМўЯТЩњГЩWЕФЛЏбЇЗНГЬЪНЮЊ______________ЁЃ
ЃЈ4ЃЉGЪЧTЕФЭЌЗжвьЙЙЬхЃЌGФмКЭЬМЫсФЦЗДгІВњЩњЦјЬхЧвЗжзгжаКЌга-NH2(АБЛљ),GЕФЭЌЗжвьЙЙЬхга_________жж(ВЛПМТЧСЂЬхНсЙЙ)ЦфжадкКЫЦЦЙВеётаГЩЯЗхЕФУцЛ§БШЮЊ1:2:2:2:2ЕФНсЙЙМђЪНЮЊ___________
ЃЈ5ЃЉЩшМЦГівдмдввЯЉЮЊжївЊдСЯжЦБИвЉЮяжаМфЬх( )ЕФКЯГЩТЗЯпЃЈЮоЛњЪдМСШЮбЁЃЉЃЌКЯГЩТЗЯпГЃгУЕФБэЪОЗНЪНЮЊ:
)ЕФКЯГЩТЗЯпЃЈЮоЛњЪдМСШЮбЁЃЉЃЌКЯГЩТЗЯпГЃгУЕФБэЪОЗНЪНЮЊ: ![]() ЁЃ________________
ЁЃ________________
ЁОД№АИЁП2,3-ЖўМзЛљ-2-ЖЁДМ єЪЛљ ![]() ЯћШЅЗДгІ
ЯћШЅЗДгІ 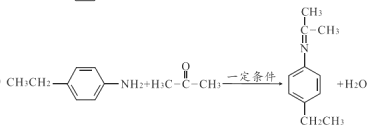 17
17 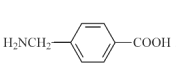 Лђ
Лђ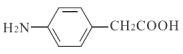
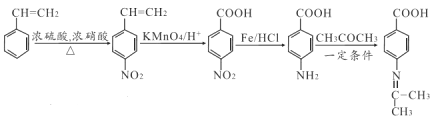
ЁОНтЮіЁП
вђЮЊXЕФЗжзгЪНЮЊC6H14O1molЃЌ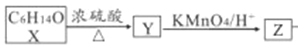 ЃЌYЭъШЋЗДгІЩњГЩ2molZ,ЧвдкМгШШЬѕМўЯТZВЛФмКЭаТжЦЧтбѕЛЏЭзЧвКЗДгІЃЌЫљвджЊXЕФУћГЦЮЊ. 2,3-ЖўМзЛљ-2-ЖЁДМЃЛZЕФНсЙЙМђЪНЮЊ
ЃЌYЭъШЋЗДгІЩњГЩ2molZ,ЧвдкМгШШЬѕМўЯТZВЛФмКЭаТжЦЧтбѕЛЏЭзЧвКЗДгІЃЌЫљвджЊXЕФУћГЦЮЊ. 2,3-ЖўМзЛљ-2-ЖЁДМЃЛZЕФНсЙЙМђЪНЮЊ![]() ЃЌZжаЙйФмЭХУћГЦЮЊєЪЛљЁЃД№АИЃК2,3-ЖўМзЛљ-2-ЖЁДМ єЪЛљЁЃ
ЃЌZжаЙйФмЭХУћГЦЮЊєЪЛљЁЃД№АИЃК2,3-ЖўМзЛљ-2-ЖЁДМ єЪЛљЁЃ
ЃЈ2ЃЉRЪЧБНЕФЭЌЯЕЮяЃЌФІЖћжЪСПЮЊ106g/mol,RЕФвЛТШДњЮяга5жжВЛЭЌНсЙЙЃЌЫљвдRЕФНсЙЙЮЊ![]() ЃЌTЮЊвЛЯѕЛљЛЏКЯЮяЃЛHЗжзгдкКЫДХББеёЧтЦзЩЯга5ИіЗхЃЌЫљвдTЕФНсЙЙМђЪНЮЊ
ЃЌTЮЊвЛЯѕЛљЛЏКЯЮяЃЛHЗжзгдкКЫДХББеёЧтЦзЩЯга5ИіЗхЃЌЫљвдTЕФНсЙЙМђЪНЮЊ![]() ЁЃгЩ
ЁЃгЩ XЮЊ2,3-ЖўМзЛљ-2-ЖЁДМЃЌЫљвдXзЊЛЏЮЊYЕФЗДгІРраЭЪЧЯћШЅЗДгІЁЃ
XЮЊ2,3-ЖўМзЛљ-2-ЖЁДМЃЌЫљвдXзЊЛЏЮЊYЕФЗДгІРраЭЪЧЯћШЅЗДгІЁЃ
ЃЈ3ЃЉгЩЩЯЪіЗжЮіжЊZЕФНсЙЙМђЪНЮЊ![]() КЭHЕФНсЙЙМђЪН
КЭHЕФНсЙЙМђЪН![]() дквЛЖЈЬѕМўЯТЩњГЩWЕФЛЏбЇЗНГЬЪНЮЊЃК
дквЛЖЈЬѕМўЯТЩњГЩWЕФЛЏбЇЗНГЬЪНЮЊЃК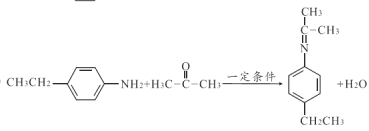
ЃЈ4ЃЉTЕФНсЙЙМђЪНЮЊ![]() ЃЌGЪЧTЕФЭЌЗжвьЙЙЬхЃЌ
ЃЌGЪЧTЕФЭЌЗжвьЙЙЬхЃЌ
GФмКЭЬМЫсИДЕФЗДгІВњЩњЦјЬхШеЗжзгжаКЌга-NH2(АБЛљ),GЕФЭЌЗжвьЙЙЬхга17жжЃЌЦфжадкКЫЦЦЙВеётаГЩЯЗхЕФУцЛ§БШЮЊ1:2:2:2:2ЕФНсЙЙМђЪНЮЊ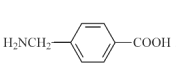 Лђ
Лђ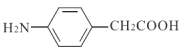 ЁЃ
ЁЃ
ЃЈ5ЃЉвдмдввЯЉЮЊжївЊдСЯжЦБИвЉЮяжаМфЬх( )ЕФКЯГЩТЗЯпЮЊ:
)ЕФКЯГЩТЗЯпЮЊ: 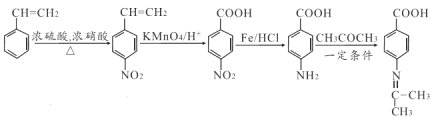 ЁЃ
ЁЃ

 УћЪІжИЕМЦкФЉГхДЬОэЯЕСаД№АИ
УћЪІжИЕМЦкФЉГхДЬОэЯЕСаД№АИ ПЊаФЭмПкЫуЬтПЈЯЕСаД№АИ
ПЊаФЭмПкЫуЬтПЈЯЕСаД№АИ