��Ŀ����
����Ŀ���ڱ���Ϊ���ִ���ҵ���������˼�����ά����������ҵ�ϳ���ͭ������(��Ҫ�ɷ���Cu2Te����Ag��Au������)Ϊԭ����ȡ�ڲ����ս����乤���������£�
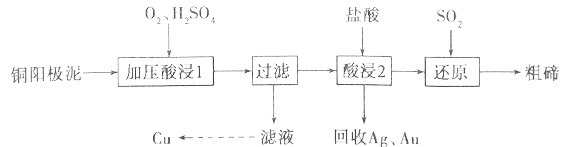
��֪��TeO2���������������ˮ�������ڽ�Ũ��ǿ���ǿ��ش��������⣺
��1������ѹ���1�������н���Ԫ��ת����TeO2��Ӧ������Һ��pHΪ4.5~5.0����Ȳ��ܹ��ߣ�ԭ����__________________________����������ѹ����Ŀ����______________________��д������ѹ���1��������Cu2Te������Ӧ�Ļ�ѧ����ʽ_________________________________��
��2�������2��ʱ�¶ȹ���ʹ�ڵĽ����ʽ��ͣ�ԭ��Ϊ______________________��Ҫ��Ag��Au�з����Au����������յ�Ag��Au�м�����Լ���______________________��
��3��д������ԭ����Ӧ�з��������ӷ���ʽ_________________________________��
��4����ҵ����һ����ȡ�ڵķ����ǽ�ͭ�������ڿ����б��գ�ʹ��ת����TeO2���ټ�NaOH�������ʯīΪ�缫�����Һ���Te���������������ĵ缫��ӦʽΪ_________________________________��
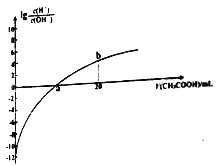
��5��25��ʱ����1mol/L��Na2TeO3��Һ�еμ����ᣬ����ҺpHֵԼΪ6ʱ����ʱ��Һ��c(TeO32��)��c(HTeO3��)=___________(��֪��H2TeO3��Ka1=1.0��10��3��Ka2=2.0��10��8)
���𰸡���Һ���Թ�ǿ��TeO2��������ᷴӦ������Ԫ����ʧ ����O2Ũ��,�ӿ췴Ӧ���ʣ���߽����� Cu2Te+2O2+2H2SO4![]() 2CuSO4+TeO2+2H2O (2��) �¶����ߣ�����ӷ�����Ӧ��Ũ�Ƚ��ͣ����½����ʽ��� ϡ���� Te4+��2SO2��4H2O==Te����2SO42-��8H+ TeO32-+3H2O+4e-=Te+6OH- 1�U50
2CuSO4+TeO2+2H2O (2��) �¶����ߣ�����ӷ�����Ӧ��Ũ�Ƚ��ͣ����½����ʽ��� ϡ���� Te4+��2SO2��4H2O==Te����2SO42-��8H+ TeO32-+3H2O+4e-=Te+6OH- 1�U50
��������
TeO2����ˮ�������ڽ�Ũ��ǿ���ǿ���ҵ�ϳ���ͭ�����ࣨ��Ҫ����TeO2������Ag��Au��Ϊԭ���Ʊ������ڣ���������Ҫ����TeO2������Ag��Au,������������TeO2�ܽ⣬���˵õ�����Ϊ�����ڼ��Ag��Au����Һ�м����������,���˵õ�TeO2�����������ܽ��ͨ���������ԭ�õ������ڡ�
��1������ѹ���1�������н���Ԫ��ת����TeO2��Ӧ������Һ��pHΪ4.5~5.0����Ȳ��ܹ��ߣ�ԭ���ǣ�TeO2�������������Һ���Թ�ǿTeO2�������H2SO4��Ӧ�����°�Ԫ����ʧ������ѹ���������̵����ӷ���ʽΪ��Cu2Te+2O2+4H��=2Cu2��+TeO2+2H2O����������ѹ����Ŀ���ǣ�����O2Ũ��,�ӿ췴Ӧ���ʣ���߽�����������ѹ���1��������Cu2Te������Ӧ�Ļ�ѧ����ʽΪCu2Te+2O2+2H2SO4![]() 2CuSO4+TeO2+2H2O��
2CuSO4+TeO2+2H2O��
��2�������2��ʱ�¶ȹ���ʹ�ڵĽ����ʽ��ͣ���Ϊ�¶����ᵼ������ӷ����࣬��Ӧ��Ũ�Ƚ��ͣ����½����ʽ��ͣ�Ҫ��Ag��Au�з����Au����Ϊ�����������ᣬ�����������ᣬ�ʿ�������յ�Ag��Au�м�����Լ���ϡ���ᡣ
��3������ԭ����Ӧ��������Te4+��ԭΪTe�����������ӷ���ʽTe4+��2SO2��4H2O==Te����2SO42-��8H+��
��4����ҵ�ϻ����Խ�ͭ���������ա������õ�Na2TeO3��Ȼ��ͨ�����ķ����õ������ڡ���֪���ʱ�ĵ缫��Ϊʯī������������ԭ��Ӧ��TeԪ�ػ��ϼ۴�+4��Ϊ0�ۣ��������ĵ缫��ӦʽΪTeO32��+3H2O+4e��=Te+6OH����
��5��25��ʱ����1mol/L��Na2TeO3��Һ�еμ����ᣬ����ҺpHֵԼΪ6ʱ��c��H����=1.0��10-6mol��L��1��Ka2=c(TeO32��)��c(H��)/c(HTeO3��)=2.0��10-8����c��HTeO3������c��TeO32����=c��H������Ka2=1.0��10-6��2.0��10-8=50��1��c(TeO32��)��c(HTeO3��)=1��50.

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ����ͼ����ѧ��ѧ�г����ڻ����ķ�����ᴿ��װ�ã������װ�ûش����⣺
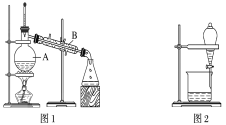
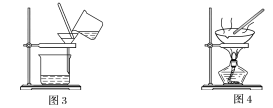
(1)װ��ͼ1��B��������________________��ͼ2��©����������________________��A��һ��Ҫ�������Ƭ����������__________________________�����й�������ʵ�����˵��һ����ȷ����_��
A��ͼ1ʵ���У�����һ��ʱ�����δ�������Ƭ��Ӧ�������ӣ��Է�����Σ��
B��ͼ2ʵ���У�Ӧ���������л��ܼ����¶˵����зų�
C��ͼ3ʵ���У������ò�������©���н��裬�Լӿ�����ٶ�
D��ͼ4ʵ���У����������н϶��������ʱ����ֹͣ����
(2)����һƿA��B�Ļ��Һ����֪���ǵ��������±���
���� | �۵�/�� | �е�/�� | �ܶ�/g��cm��3 | �ܽ��� |
A | ��11.5 | 198 | 1.11 | A��B���ܣ��Ҿ�������ˮ�;ƾ� |
B | 17.9 | 290 | 1.26 |
�ݴ˷�������A��B������ѡ����ͼ�е�ͼ________________��ʾ������
(3)��ͼ2��ʾʵ���У����÷ֲ�������֪����һ��Һ������ˮ�����������һ�ּ����жϷ�����____��