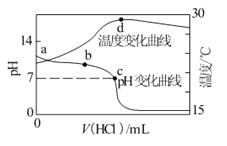
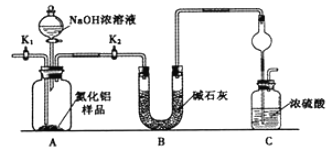
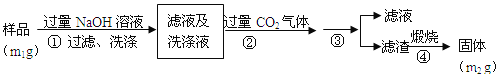
题目内容
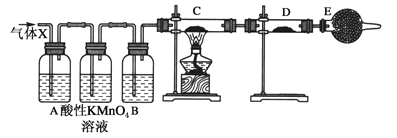
【题目】某课外活动小组的同学,在实验室做锌与浓硫酸反应的实验中,甲同学认为产生的气体是二氧化硫,而乙同学认为除二氧化硫气体外,还可以产生氢气。为了验证甲、乙两位同学的判断是否正确,丙同学设计了如图所示实验装置(锌与浓硫酸共热时产生的气体为X,且该装置略去,且洗气瓶中高锰酸钾溶液足量)。试回答:
(1)上述反应中生成二氧化硫的化学方程式为:__________________。
(2)乙同学认为还可能产生氢气的理由是:_____________________。
(3)丙同学在安装好装置后,必不可少的一步操作是:________________。
(4)A中加入的试剂可能是____________,作用是_______________;B中加入的试剂可能是________,作用是_____________;E中加入的试剂可能是_____________,作用是________________。
(5)可以证明气体X中含有氢气的实验现象是:C中:______________,D中:_______________。
【答案】Zn+2H2SO4(浓)![]() ZnSO4+SO2↑+2H2O硫酸変稀与Zn反应生成H2检查装置的气密性品红溶液检验SO2的存在浓硫酸吸收水蒸气碱石灰防止空气中的水蒸气进入D装置C中黑色粉末变红D中白色粉末变蓝
ZnSO4+SO2↑+2H2O硫酸変稀与Zn反应生成H2检查装置的气密性品红溶液检验SO2的存在浓硫酸吸收水蒸气碱石灰防止空气中的水蒸气进入D装置C中黑色粉末变红D中白色粉末变蓝
【解析】
(1)锌和浓硫酸反应是二氧化硫、硫酸锌和水,反应的化学方程式为Zn+2H2SO4(浓)![]() ZnSO4+SO2↑+2H2O,故答案为:Zn+2H2SO4(浓)
ZnSO4+SO2↑+2H2O,故答案为:Zn+2H2SO4(浓)![]() ZnSO4+SO2↑+2H2O;
ZnSO4+SO2↑+2H2O;
(2)反应时浓H2SO4 浓度逐渐变稀,Zn与稀H2SO4 反应可产生H2,反应的化学方程式为:Zn+H2SO4=ZnSO4+H2↑;故答案为:硫酸变稀与Zn反应生成H2;
(3)该装置是气体验证实验,所以需要装置气密性完好,实验开始先检验装置的气密性,故答案为:检查装置的气密性;
(4)分析装置图可知,生成的气体中有二氧化硫和氢气,所以装置A是验证二氧化硫存在的装置,选品红溶液进行验证;通过高锰酸钾溶液除去二氧化硫,通过装置B中的浓硫酸除去水蒸气,利用氢气和氧化铜反应生成铜和水蒸气,所以利用装置D中 的无水硫酸铜检验水的生成,为避免空气中的水蒸气影响D装置中水的检验,装置E中需要用碱石灰,故答案为:品红溶液;检测SO2;浓H2SO4;吸收水蒸气;碱石灰;防止空气中的水蒸气进入D中;
(5)证明生成的气体中含有水蒸气的现象,C装置中黑色氧化铜变为红色铜,D装置中白色硫酸铜变为蓝色,故答案为:黑色粉末变成红色;白色粉末变成蓝色。